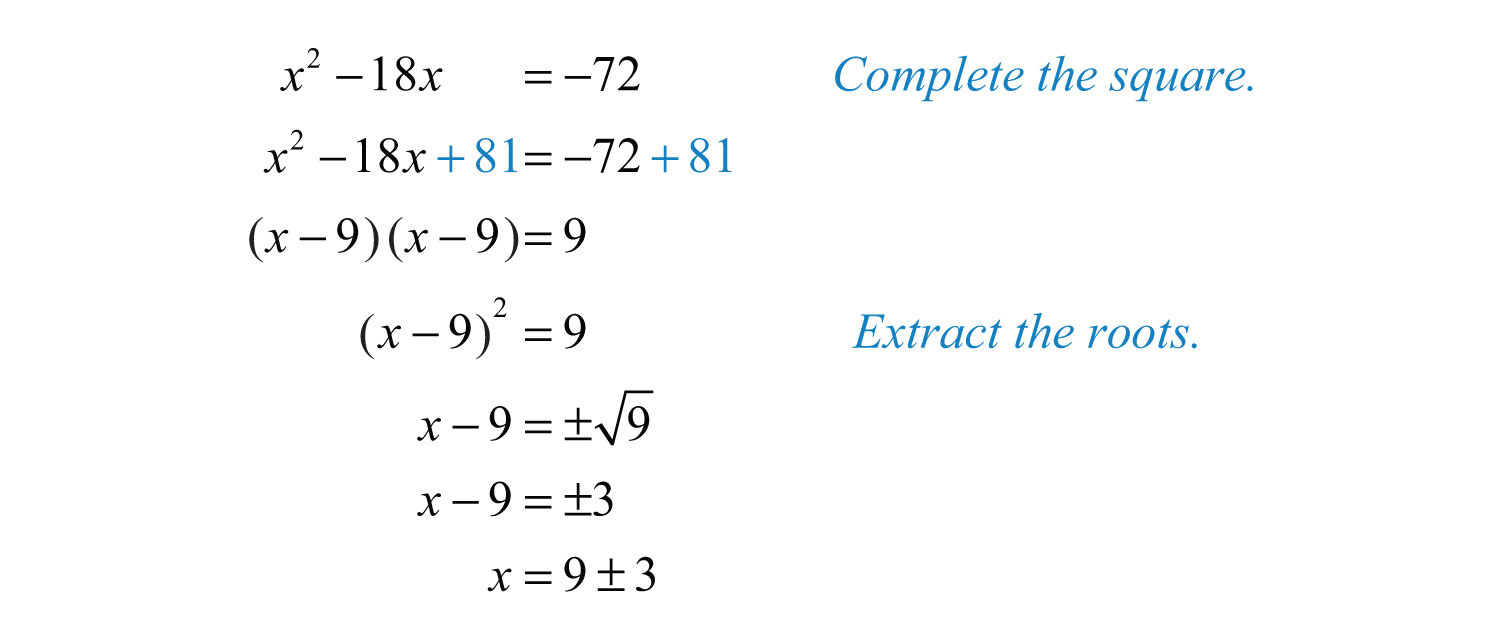5 Ways to Master Writing Chemical Equations

Writing chemical equations is a crucial skill for chemistry students and professionals alike. A well-written chemical equation provides a clear and concise representation of a chemical reaction, allowing for efficient communication and understanding of complex chemical processes. In this article, we will explore five ways to master writing chemical equations.
Understanding the Basics
Before diving into the ways to master writing chemical equations, it’s essential to understand the basics. A chemical equation is a symbolic representation of a chemical reaction, where reactants are converted into products. The equation consists of reactants on the left side and products on the right side, separated by an arrow (→). The arrow indicates the direction of the reaction.
1. Balance Your Equations
Balancing chemical equations is a critical step in writing accurate equations. A balanced equation ensures that the number of atoms of each element is the same on both the reactant and product sides. To balance an equation, follow these steps:
- Write the unbalanced equation with the reactants on the left and products on the right.
- Count the number of atoms of each element on both sides.
- Add coefficients (numbers in front of the formulas of reactants or products) to balance the equation.
- Check that the number of atoms of each element is the same on both sides.
Example:
Unbalanced equation: Na + O2 → Na2O Balanced equation: 4Na + O2 → 2Na2O
📝 Note: When balancing equations, it's essential to add coefficients, not subscripts. Changing subscripts can alter the chemical formula and lead to incorrect equations.
2. Use Correct Formatting
Proper formatting is crucial for writing clear and readable chemical equations. Follow these guidelines:
- Use the correct arrow (→) to separate reactants and products.
- Write reactants on the left and products on the right.
- Use coefficients (numbers in front of the formulas of reactants or products) to balance the equation.
- Use subscripts (small numbers to the right of the chemical symbol) to indicate the number of atoms of an element in a molecule.
- Use parentheses to group reactants or products that appear together.
Example:
2H2 + O2 → 2H2O
3. Identify and Write the States of Matter
States of matter (solid, liquid, gas, or aqueous) are essential components of chemical equations. Identify the states of matter for each reactant and product, and write them in parentheses after the formula.
Example:
CaCO3 (s) + 2HCl (aq) → CaCl2 (aq) + H2O (l) + CO2 (g)
4. Write Equations for Different Types of Reactions
Chemical reactions can be classified into several types, including synthesis, decomposition, single displacement, double displacement, and combustion reactions. Understand the characteristics of each type of reaction and practice writing equations for each.
Example:
Synthesis reaction: 2H2 + O2 → 2H2O Decomposition reaction: 2H2O → 2H2 + O2 Single displacement reaction: Zn + CuSO4 → ZnSO4 + Cu
5. Practice, Practice, Practice!
Writing chemical equations is a skill that requires practice to master. Practice writing equations for different types of reactions, and balance them to ensure accuracy. You can find online resources, such as worksheets and quizzes, to help you practice.
Additional Tips:
- Use online tools, such as equation balancers, to help you balance equations.
- Check your work by counting the number of atoms of each element on both sides of the equation.
- Practice writing equations for different types of reactions to improve your understanding of chemical processes.
By following these five ways to master writing chemical equations, you’ll become proficient in writing accurate and clear chemical equations. Remember to practice regularly to reinforce your skills.
Chemical equations are a fundamental language of chemistry, and mastering them is essential for success in the field. By applying these tips and practicing regularly, you’ll become proficient in writing chemical equations and improve your understanding of chemical processes.
Summary:
In this article, we explored five ways to master writing chemical equations: balancing equations, using correct formatting, identifying and writing the states of matter, writing equations for different types of reactions, and practicing regularly. By applying these tips and practicing regularly, you’ll become proficient in writing accurate and clear chemical equations.
What is the purpose of balancing chemical equations?
+Balancing chemical equations ensures that the number of atoms of each element is the same on both the reactant and product sides, providing an accurate representation of the chemical reaction.
How do I balance a chemical equation?
+To balance a chemical equation, count the number of atoms of each element on both sides, and add coefficients (numbers in front of the formulas of reactants or products) to balance the equation.
What are the different types of chemical reactions?
+Chemical reactions can be classified into several types, including synthesis, decomposition, single displacement, double displacement, and combustion reactions.
Related Terms:
- Writing word equations Worksheet
- Balancing chemical equations Worksheet PDF