Chemical vs Physical Properties and Changes Explained
Understanding the Difference Between Chemical and Physical Properties and Changes
In the world of chemistry, properties and changes are fundamental concepts that help us understand the behavior of matter. However, it’s essential to differentiate between chemical and physical properties and changes, as they are often confused with one another. In this article, we’ll delve into the definitions, examples, and explanations of chemical and physical properties and changes, highlighting their distinct characteristics.
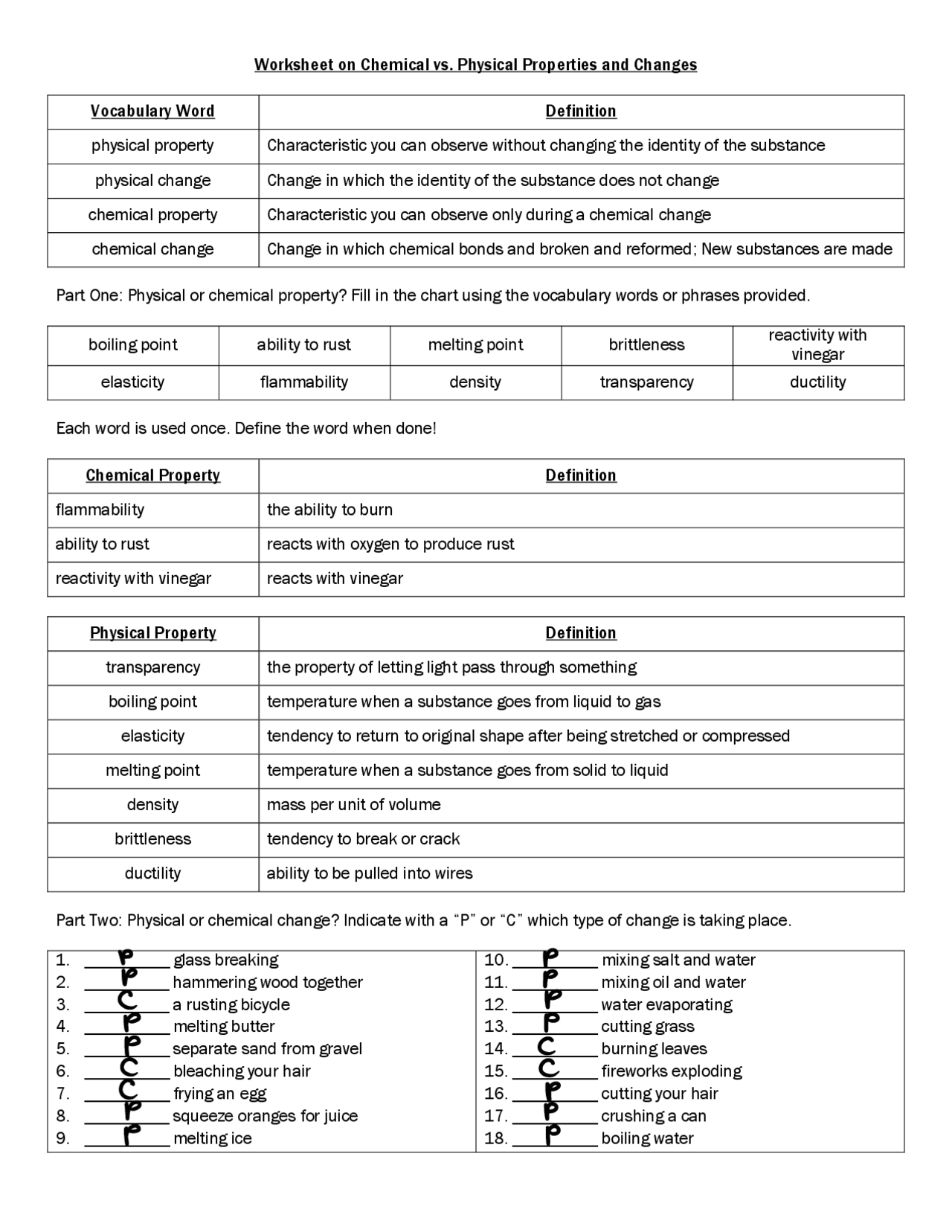
Chemical Properties
Chemical properties are characteristics of a substance that can be observed or measured when it undergoes a chemical change. These properties are often used to identify a substance and predict its behavior in different chemical reactions. Examples of chemical properties include:
- Flammability: the ability of a substance to burn in the presence of oxygen
- Reactivity: the ability of a substance to react with other substances to form new compounds
- Toxicity: the ability of a substance to cause harm or death to living organisms
These properties are often used to identify a substance and predict its behavior in different chemical reactions.
Physical Properties
Physical properties, on the other hand, are characteristics of a substance that can be observed or measured without changing its chemical composition. These properties are often used to describe the physical state or appearance of a substance. Examples of physical properties include:
- Color: the visible light that is reflected or transmitted by a substance
- Odor: the smell of a substance
- Melting point: the temperature at which a substance changes from a solid to a liquid state
- Boiling point: the temperature at which a substance changes from a liquid to a gas state
These properties are often used to describe the physical state or appearance of a substance.
Chemical Changes
Chemical changes, also known as chemical reactions, occur when a substance undergoes a reaction that changes its chemical composition. These changes are often irreversible and result in the formation of new substances. Examples of chemical changes include:
- Combustion: the reaction of a substance with oxygen to produce heat and light
- Oxidation: the reaction of a substance with oxygen to form a new compound
- Synthesis: the reaction of two or more substances to form a new compound
These changes are often used to produce new substances or energy.
Physical Changes
Physical changes, on the other hand, occur when a substance changes its physical state or appearance without changing its chemical composition. These changes are often reversible and result in the formation of the same substance in a different state. Examples of physical changes include:
- Melting: the change of a substance from a solid to a liquid state
- Freezing: the change of a substance from a liquid to a solid state
- Vaporization: the change of a substance from a liquid to a gas state
These changes are often used to describe the physical state or appearance of a substance.
Examples and Illustrations
To illustrate the difference between chemical and physical properties and changes, let’s consider the following examples:
- Burning of Wood: When wood is burned, it undergoes a chemical change, releasing heat and light. The wood is converted into ash, carbon dioxide, and water vapor. This is an example of a chemical change.
- Melting of Ice: When ice is melted, it undergoes a physical change, changing from a solid to a liquid state. The chemical composition of the ice remains the same. This is an example of a physical change.
📝 Note: The distinction between chemical and physical properties and changes is crucial in understanding the behavior of matter and predicting its behavior in different situations.
Importance of Understanding Chemical and Physical Properties and Changes
Understanding the difference between chemical and physical properties and changes is essential in various fields, including chemistry, physics, biology, and engineering. This knowledge helps us:
- Identify substances: by understanding their chemical and physical properties
- Predict behavior: by understanding how substances will react in different situations
- Design processes: by understanding how to manipulate substances to produce desired outcomes
- Ensure safety: by understanding the potential hazards associated with substances and their reactions
In conclusion, understanding the difference between chemical and physical properties and changes is crucial in understanding the behavior of matter and predicting its behavior in different situations. By recognizing the distinct characteristics of each, we can better identify substances, predict behavior, design processes, and ensure safety.
What is the main difference between chemical and physical properties?
+The main difference between chemical and physical properties is that chemical properties are characteristics of a substance that can be observed or measured when it undergoes a chemical change, while physical properties are characteristics of a substance that can be observed or measured without changing its chemical composition.
What is an example of a chemical change?
+An example of a chemical change is the burning of wood, which releases heat and light and converts the wood into ash, carbon dioxide, and water vapor.
Why is it important to understand the difference between chemical and physical properties and changes?
+Understanding the difference between chemical and physical properties and changes is essential in various fields, including chemistry, physics, biology, and engineering, as it helps us identify substances, predict behavior, design processes, and ensure safety.
Related Terms:
- Physical and chemical changes PDF
- Physical and chemical properties practice