Separating Mixtures Made Easy
Introduction to Separating Mixtures
In our daily lives, we often encounter various mixtures of substances that need to be separated. Whether it’s a mixture of sand and water, a combination of different metals, or a blend of various chemicals, separating mixtures is an essential process in many fields, including chemistry, biology, and environmental science. The process of separating mixtures can be complex and requires a thorough understanding of the properties of the substances involved. In this article, we will explore the different methods used to separate mixtures, the principles behind them, and some practical examples.
Methods for Separating Mixtures
There are several methods used to separate mixtures, each with its own advantages and limitations. Some of the most common methods include:
- Filtration: This method involves passing the mixture through a filter, which allows the smaller particles to pass through while retaining the larger particles.
- Decantation: This method involves carefully pouring the liquid from one container to another, leaving the sediment or particles behind.
- Evaporation: This method involves heating the mixture to evaporate the liquid, leaving the solid particles behind.
- Crystallization: This method involves cooling the mixture to form crystals, which can then be separated from the remaining liquid.
- Magnetic Separation: This method involves using a magnet to attract and separate magnetic particles from non-magnetic particles.
- Chromatography: This method involves using a stationary phase and a mobile phase to separate mixtures based on their chemical properties.
Principles of Separation
Each separation method is based on specific principles, which are essential to understand in order to apply the methods effectively. Some of the key principles include:
- Density: Separation methods such as decantation and filtration rely on the difference in density between the particles and the liquid.
- Solubility: Separation methods such as crystallization and evaporation rely on the difference in solubility between the particles and the liquid.
- Magnetic Properties: Separation methods such as magnetic separation rely on the difference in magnetic properties between the particles.
- Chemical Properties: Separation methods such as chromatography rely on the difference in chemical properties between the particles.
Practical Examples
Let’s take a look at some practical examples of separating mixtures:
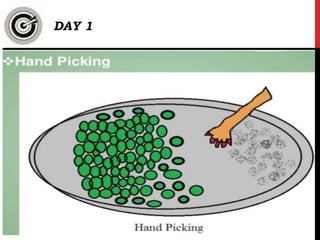
- Separating Sand and Water: A mixture of sand and water can be separated using filtration. The sand particles are retained by the filter, while the water passes through.
- Separating Iron from Copper: A mixture of iron and copper can be separated using magnetic separation. The iron particles are attracted to the magnet, while the copper particles are not.
- Separating Salt from Water: A mixture of salt and water can be separated using evaporation. The water is evaporated, leaving the salt behind.
🔍 Note: The choice of separation method depends on the specific properties of the substances involved and the desired outcome.
Challenges and Limitations
While separating mixtures can be a straightforward process, there are also challenges and limitations to consider. Some of the common challenges include:
- Incomplete Separation: In some cases, the separation method may not be able to completely separate the mixture, resulting in a partial separation.
- Contamination: The separation process can sometimes introduce contaminants into the separated substances.
- Scalability: Separation methods may not be scalable for large quantities of mixtures.
Conclusion
Separating mixtures is an essential process in many fields, and understanding the different methods and principles involved is crucial for effective separation. By choosing the right separation method and considering the challenges and limitations, we can achieve efficient and accurate separation of mixtures.
What is the most common method of separating mixtures?
+Filtration is one of the most common methods of separating mixtures.
What is the principle behind magnetic separation?
+Magnetic separation relies on the difference in magnetic properties between the particles.
What are some common challenges in separating mixtures?
+Some common challenges include incomplete separation, contamination, and scalability issues.