5 Key Concepts of Atomic Structure and the Periodic Table
Unlocking the Secrets of Atomic Structure and the Periodic Table
Atomic structure and the periodic table are fundamental concepts in chemistry that help us understand the building blocks of matter and how they interact with each other. The periodic table is a powerful tool that organizes elements based on their atomic structure, allowing us to identify patterns and relationships between elements. In this article, we will explore five key concepts of atomic structure and the periodic table, providing a comprehensive understanding of these essential concepts.
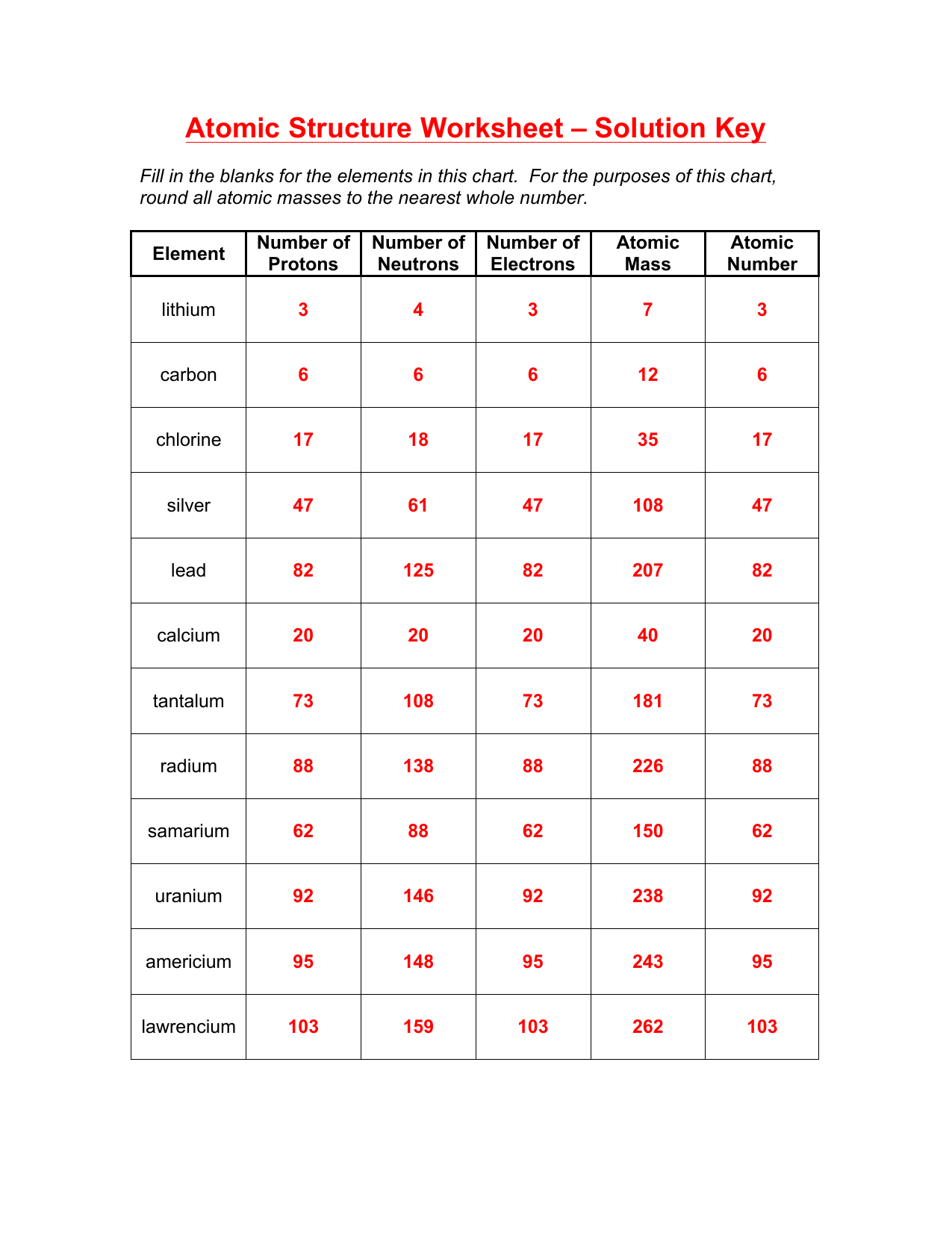
1. Atomic Number and Atomic Mass
The atomic number of an element is the number of protons present in the nucleus of an atom, while the atomic mass is the total number of protons and neutrons present in the nucleus. The atomic number determines the identity of an element, and each element has a unique atomic number. On the other hand, the atomic mass is used to calculate the average mass of an element, taking into account the naturally occurring isotopes of that element.
🔍 Note: The atomic number is a fundamental property of an element, while the atomic mass is a derived property that depends on the isotopic composition of the element.
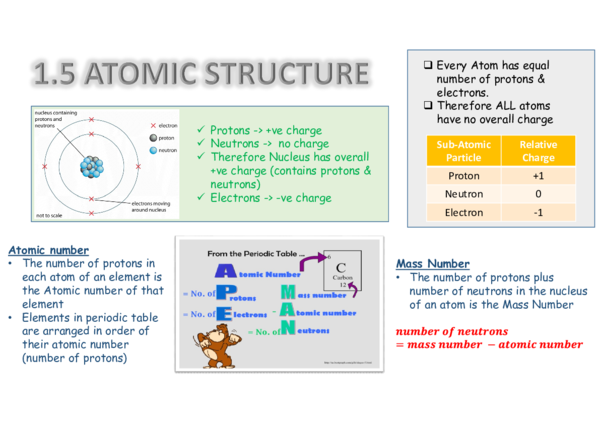
2. Electron Configuration and Energy Levels
Electron configuration refers to the arrangement of electrons in an atom, which is determined by the energy levels or shells that the electrons occupy. The energy levels are quantized, meaning that electrons can only occupy specific energy levels, and each energy level has a limited capacity. The electron configuration of an atom determines its chemical properties, such as reactivity and electron affinity.
| Energy Level | Electron Capacity |
|---|---|
| 1s | 2 |
| 2s | 2 |
| 2p | 6 |
| 3s | 2 |
| 3p | 6 |
| 3d | 10 |
3. Periodic Trends and Blocks
The periodic table is organized into blocks, which are groups of elements with similar electron configurations. The blocks are further divided into periods, which are rows of elements with the same number of electron shells. The periodic trends, such as atomic radius, electronegativity, and ionization energy, are related to the electron configuration and energy levels of the elements.
- s-block elements: Group 1 and 2 elements, which have one or two electrons in their outermost energy level.
- p-block elements: Group 13-18 elements, which have three or more electrons in their outermost energy level.
- d-block elements: Group 3-12 elements, which have partially filled d subshells.
- f-block elements: Lanthanides and actinides, which have partially filled f subshells.
4. Valence Electrons and Chemical Reactivity
Valence electrons are the electrons in the outermost energy level of an atom, which participate in chemical bonding. The number of valence electrons determines the chemical reactivity of an element, and elements with similar numbers of valence electrons exhibit similar chemical properties.
- Metals: Typically have few valence electrons and tend to lose electrons to form cations.
- Nonmetals: Typically have many valence electrons and tend to gain electrons to form anions.
- Metalloids: Have intermediate numbers of valence electrons and exhibit intermediate chemical properties.
5. Periodic Table Families and Groups
The periodic table is divided into families or groups, which are columns of elements with similar chemical properties. The families are determined by the number of electrons in the outermost energy level and the electron configuration of the elements.
- Alkali metals: Group 1 elements, which have one electron in their outermost energy level.
- Noble gases: Group 18 elements, which have full outer energy levels.
- Halogens: Group 17 elements, which have seven electrons in their outermost energy level.
By understanding these five key concepts of atomic structure and the periodic table, we can unlock the secrets of the periodic table and appreciate the beauty and logic of the organization of elements.
In summary, the key concepts of atomic structure and the periodic table are fundamental to understanding the properties and behavior of elements. By mastering these concepts, we can gain a deeper appreciation for the periodic table and its role in chemistry.
What is the significance of the atomic number in the periodic table?
+The atomic number determines the identity of an element and is a fundamental property of an element.
How do electron configuration and energy levels relate to chemical properties?
+The electron configuration of an atom determines its chemical properties, such as reactivity and electron affinity.
What are the main differences between metals, nonmetals, and metalloids?
+Metals typically have few valence electrons and tend to lose electrons, nonmetals have many valence electrons and tend to gain electrons, and metalloids have intermediate numbers of valence electrons and exhibit intermediate chemical properties.
Related Terms:
- Igcse atomic structure worksheet pdf
- Atomic structure IGCSE Notes