Atomic Basics Worksheet: Fundamentals of Atomic Structure Explained
Understanding the Atomic Basics: A Comprehensive Guide
The atomic structure is the foundation of chemistry, and understanding its basics is crucial for any student or enthusiast of the subject. In this article, we will delve into the fundamental concepts of atomic structure, exploring the key components and their relationships. By the end of this article, you will have a solid grasp of the atomic basics, enabling you to tackle more complex chemistry topics with confidence.
What is an Atom?
An atom is the smallest unit of a chemical element, and it is the building block of matter. Atoms are incredibly small, with diameters ranging from 1 to 3 angstroms (Å). Despite their tiny size, atoms are composed of even smaller particles called subatomic particles.
Subatomic Particles: Protons, Neutrons, and Electrons
There are three primary subatomic particles that make up an atom:
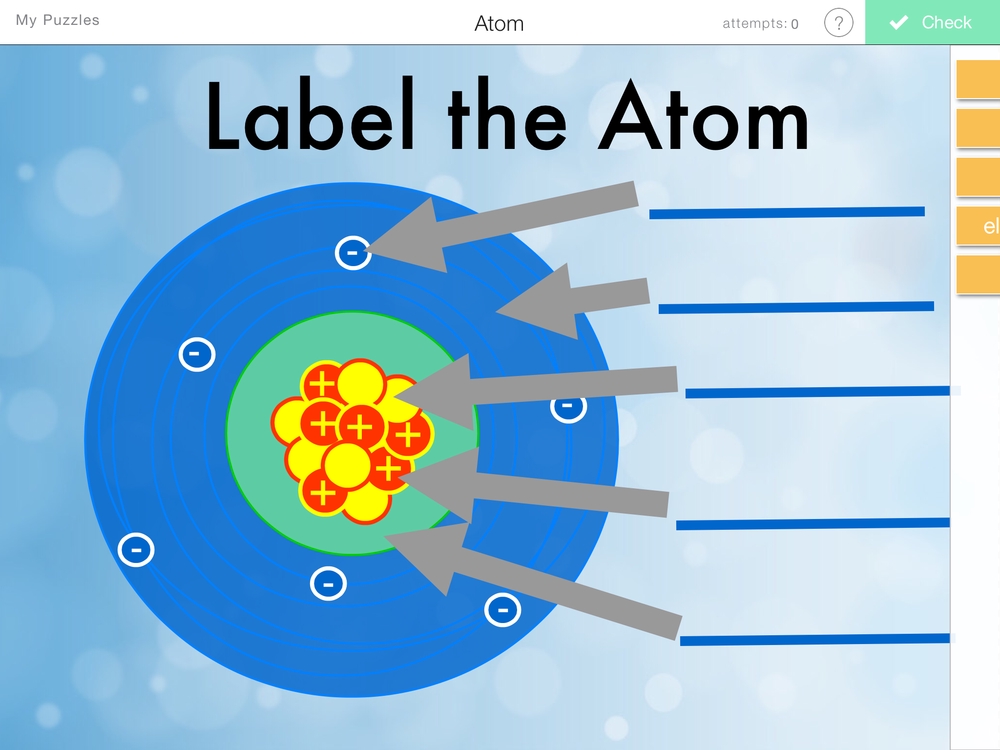
- Protons: Positively charged particles that reside in the nucleus (center) of the atom. The number of protons in an atom determines the element of an atom, and each element has a unique number of protons in its atoms.
- Neutrons: Particles with no charge that are found in the nucleus along with protons. The number of neutrons in an atom can vary, leading to different isotopes (atoms of the same element with different numbers of neutrons) of an element.
- Electrons: Negatively charged particles that orbit the nucleus of an atom. The number of electrons in a neutral atom is equal to the number of protons, and electrons are arranged in energy levels or electron shells around the nucleus.
The Nucleus: Center of the Atom
The nucleus is the central part of an atom, containing the protons and neutrons. The nucleus is incredibly dense, with the protons and neutrons packed tightly together. The number of protons in the nucleus determines the atomic number of an element, which is unique to each element.
Energy Levels and Electron Shells
Electrons are arranged in energy levels or electron shells around the nucleus. These energy levels are quantized, meaning that electrons can only occupy specific energy levels. The energy levels are filled in a specific order, with the lowest energy level filled first.
| Energy Level | Maximum Number of Electrons |
|---|---|
| 1st energy level | 2 |
| 2nd energy level | 8 |
| 3rd energy level | 18 |
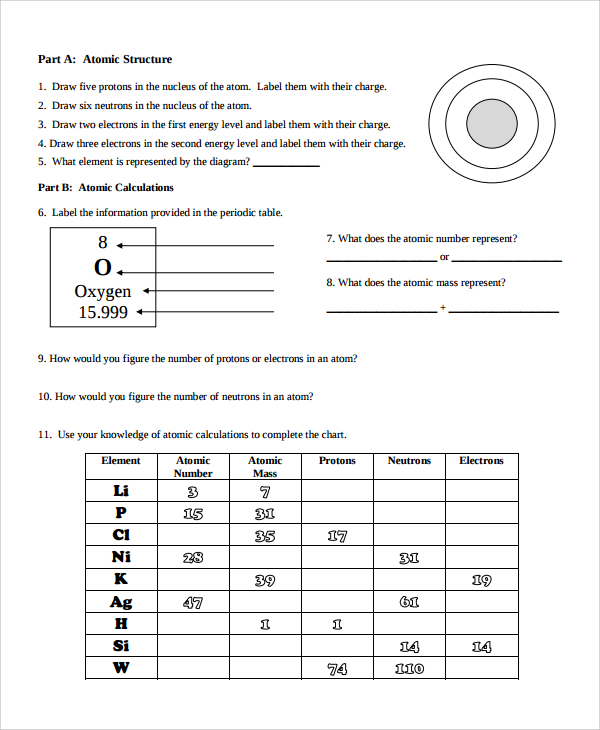
Atomic Number and Mass Number
The atomic number of an element is the number of protons in the nucleus of an atom, while the mass number is the total number of protons and neutrons. The mass number is also known as the atomic mass.
| Element | Atomic Number | Mass Number |
|---|---|---|
| Hydrogen | 1 | 1 |
| Helium | 2 | 4 |
| Oxygen | 8 | 16 |
🔍 Note: The atomic number and mass number are often represented by the symbols Z and A, respectively.
Isotopes and Ions
Isotopes are atoms of the same element with different numbers of neutrons. Ions are atoms that have gained or lost electrons, resulting in a net positive or negative charge.
| Isotope | Number of Protons | Number of Neutrons |
|---|---|---|
| Carbon-12 | 6 | 6 |
| Carbon-13 | 6 | 7 |
| Carbon-14 | 6 | 8 |
Summary
In conclusion, the atomic structure is a fundamental concept in chemistry, and understanding its basics is essential for any student or enthusiast of the subject. By grasping the concepts of subatomic particles, the nucleus, energy levels, and atomic number and mass number, you will have a solid foundation in atomic structure.
What is the difference between an atom and a molecule?
+
An atom is the smallest unit of a chemical element, while a molecule is a group of atoms bonded together.
What is the atomic number of an element?
+
The atomic number of an element is the number of protons in the nucleus of an atom, which is unique to each element.
What is the difference between an isotope and an ion?
+
An isotope is an atom of the same element with a different number of neutrons, while an ion is an atom that has gained or lost electrons, resulting in a net positive or negative charge.