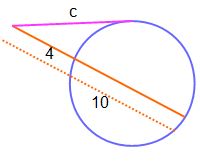
Molecular Compound Naming Worksheet Answers

Molecular Compound Naming Worksheet Answers
Molecular compounds are formed when two or more nonmetal elements combine in a fixed ratio. These compounds are typically neutral and do not conduct electricity in solution. The naming of molecular compounds is based on the names of the elements that make up the compound and the number of atoms of each element present. In this worksheet, we will provide answers to common molecular compound naming questions.
Simple Molecular Compounds
The simplest molecular compounds are formed between two nonmetal elements. The names of these compounds are derived by combining the names of the elements, with the first element taking the prefix indicating the number of atoms and the second element taking the prefix and changing the ending to -ide.
Examples:
- CO: Carbon monoxide (1 carbon, 1 oxygen)
- CO2: Carbon dioxide (1 carbon, 2 oxygen)
- N2O: Dinitrogen monoxide (2 nitrogen, 1 oxygen)
Molecular Compounds with Prefixes
When naming molecular compounds, prefixes are used to indicate the number of atoms of each element present.
- Mono-: 1
- Di-: 2
- Tri-: 3
- Tetra-: 4
- Penta-: 5
- Hexa-: 6
- Hepta-: 7
- Octa-: 8
- Nona-: 9
- Deca-: 10
Examples:
- N2O4: Dinitrogen tetroxide (2 nitrogen, 4 oxygen)
- SF6: Sulfur hexafluoride (1 sulfur, 6 fluorine)
- PCl3: Phosphorus trichloride (1 phosphorus, 3 chlorine)
Molecular Compounds with Multiple Bonds
When a molecular compound contains multiple bonds between the same two elements, the name of the compound includes the prefix indicating the number of atoms and the suffix indicating the type of bond.
- -yne: Triple bond
- -ene: Double bond
- -ane: Single bond
Examples:
- C2H2: Ethyne (2 carbon, 2 hydrogen, triple bond)
- C2H4: Ethene (2 carbon, 4 hydrogen, double bond)
- C2H6: Ethane (2 carbon, 6 hydrogen, single bond)
Common Molecular Compound Names
Some molecular compounds have common names that are widely used.
- Water: H2O
- Ammonia: NH3
- Carbon dioxide: CO2
- Methane: CH4
📝 Note: Common names are often used for simple molecular compounds, but IUPAC names should be used for more complex compounds.
Multiple Elements
When a molecular compound contains more than two nonmetal elements, the name of the compound includes the prefixes indicating the number of atoms of each element and the suffix indicating the type of bond.
Examples:
- CH4O: Methanol (1 carbon, 4 hydrogen, 1 oxygen)
- C2H5Cl: Chloroethane (2 carbon, 5 hydrogen, 1 chlorine)
📝 Note: When naming molecular compounds with multiple elements, the elements are listed in the order of their atomic number.
Summary
Molecular compound naming is based on the names of the elements and the number of atoms of each element present. Prefixes are used to indicate the number of atoms, and suffixes are used to indicate the type of bond. Common names are often used for simple molecular compounds, but IUPAC names should be used for more complex compounds.
To master molecular compound naming, practice with different combinations of elements and prefixes. Remember to use the correct suffixes to indicate the type of bond and to list the elements in the order of their atomic number.
By following these rules and guidelines, you will be able to name molecular compounds with confidence.
In conclusion, the key to molecular compound naming is understanding the prefixes and suffixes used to indicate the number of atoms and the type of bond. With practice and patience, you will become proficient in naming molecular compounds and be able to tackle more complex chemistry problems.
What is the prefix for 1 atom of an element?
+Mono-
What is the suffix for a triple bond?
+-yne
What is the common name for H2O?
+Water