5 Easy Ways to Master Stoichiometry
Stoichiometry, a branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions, can be a daunting subject for many students. However, with the right approach and strategies, mastering stoichiometry can become a breeze. In this article, we will explore 5 easy ways to master stoichiometry and improve your understanding of this fundamental concept in chemistry.
1. Understand the Basics of Chemical Reactions
Before diving into stoichiometry, it’s essential to have a solid grasp of chemical reactions. Start by reviewing the types of chemical reactions, such as synthesis, decomposition, single displacement, and double displacement reactions. Make sure you understand the concept of reactants, products, and the law of conservation of mass.
- Key Terms:
- Reactants: substances that undergo a chemical change
- Products: substances formed as a result of a chemical reaction
- Law of Conservation of Mass: matter cannot be created or destroyed in a chemical reaction
2. Learn to Balance Chemical Equations
Balancing chemical equations is a crucial step in stoichiometry. Practice balancing simple equations using the law of conservation of mass. Start with simple reactions and gradually move on to more complex ones.
- Tips:
- Count the number of atoms of each element on both the reactant and product sides
- Use coefficients (numbers in front of formulas of reactants or products) to balance the equation
- Check your work by verifying that the number of atoms of each element is equal on both sides
3. Master the Mole Concept
The mole concept is the foundation of stoichiometry. Understand the relationship between moles, mass, and number of particles. Practice converting between moles, grams, and particles.
- Key Concepts:
- 1 mole = 6.022 x 10^23 particles (atoms or molecules)
- Molar mass: mass of 1 mole of a substance (in g/mol)
- Conversions: moles → grams, grams → moles, particles → moles
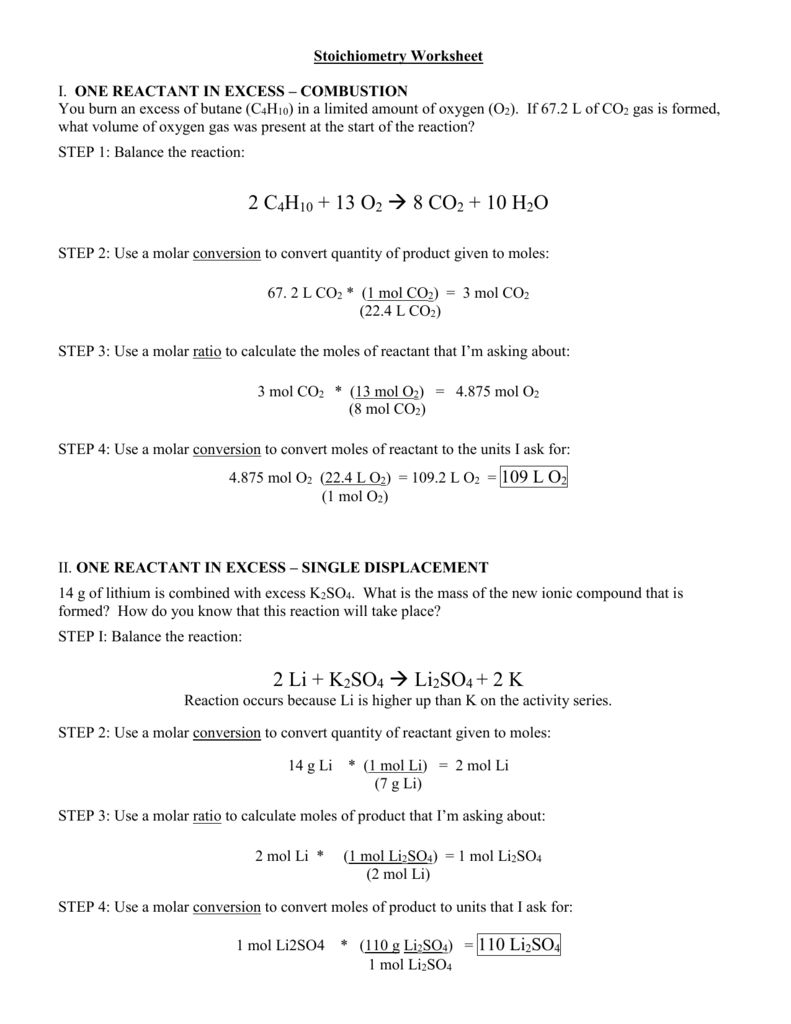
4. Practice Stoichiometry Problems
Practice is key to mastering stoichiometry. Start with simple problems, such as calculating the number of moles of a reactant or product, and gradually move on to more complex problems.
- Problem Types:
- Calculating the number of moles of a reactant or product
- Finding the mass of a reactant or product
- Calculating the volume of a gas at STP (Standard Temperature and Pressure)
- Determining the limiting reactant
5. Use Online Resources and Visual Aids
There are many online resources and visual aids available to help you master stoichiometry. Utilize online calculators, videos, and interactive simulations to supplement your learning.
- Recommended Resources:
- Khan Academy (khanacademy.org)
- Crash Course (crashcourse.com)
- Stoichiometry calculators (e.g., stoichiometrycalculator.org)
- Interactive simulations (e.g., phet.colorado.edu)
👍 Note: Consistency is key. Set aside time each day to practice and review stoichiometry concepts.
In conclusion, mastering stoichiometry requires a combination of understanding the basics of chemical reactions, learning to balance chemical equations, mastering the mole concept, practicing stoichiometry problems, and utilizing online resources and visual aids. With consistent practice and review, you’ll become proficient in stoichiometry in no time.
What is the law of conservation of mass?
+The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. The total mass of the reactants is equal to the total mass of the products.
How do I balance a chemical equation?
+To balance a chemical equation, count the number of atoms of each element on both the reactant and product sides. Use coefficients (numbers in front of formulas of reactants or products) to balance the equation. Check your work by verifying that the number of atoms of each element is equal on both sides.
What is the mole concept?
+The mole concept is the relationship between moles, mass, and number of particles. One mole is equal to 6.022 x 10^23 particles (atoms or molecules). The mole concept allows us to convert between moles, grams, and particles.
Related Terms:
- Stoichiometry Worksheet with answers pdf
- Basic Stoichiometry Worksheet
- Molarity Stoichiometry Worksheet with answers
- Stoichiometry Review Worksheet with answers
- Honors chemistry Stoichiometry Worksheet answers