3 Ways to Master Stoichiometry Percent Yield

Understanding Stoichiometry Percent Yield
Stoichiometry is a fundamental concept in chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. One of the most important aspects of stoichiometry is percent yield, which is a measure of the efficiency of a chemical reaction. In this blog post, we will explore three ways to master stoichiometry percent yield and provide tips and tricks to help you calculate it accurately.
Method 1: Understanding the Concept of Percent Yield
Percent yield is defined as the ratio of the actual yield of a product to the theoretical yield, multiplied by 100%. The actual yield is the amount of product obtained from a reaction, while the theoretical yield is the amount of product that would be obtained if the reaction were 100% efficient. To calculate percent yield, you need to know the actual yield and the theoretical yield.
Formula:
Percent Yield = (Actual Yield / Theoretical Yield) x 100%
Example:
Suppose you perform a reaction that yields 25g of product, and the theoretical yield is 30g. To calculate the percent yield, you would use the formula:
Percent Yield = (25g / 30g) x 100% = 83.3%
This means that the reaction is 83.3% efficient.
Method 2: Mastering the Calculation of Theoretical Yield
To calculate the theoretical yield, you need to know the balanced chemical equation, the number of moles of each reactant, and the molar mass of each product. Here are the steps to follow:
- Write the balanced chemical equation.
- Calculate the number of moles of each reactant.
- Determine the limiting reactant (the reactant that is consumed first).
- Calculate the number of moles of product produced from the limiting reactant.
- Multiply the number of moles of product by the molar mass to get the theoretical yield.
Example:
Suppose you have the following reaction:
2H2 + O2 → 2H2O
You start with 10g of H2 and 20g of O2. To calculate the theoretical yield of H2O, you would follow these steps:
- Calculate the number of moles of H2 and O2.
- Determine the limiting reactant (in this case, H2).
- Calculate the number of moles of H2O produced from the limiting reactant.
- Multiply the number of moles of H2O by the molar mass to get the theoretical yield.
💡 Note: Make sure to use the correct units when calculating the theoretical yield. In this example, we used grams of H2 and O2, but we need to convert them to moles first.
Method 3: Using Stoichiometry Tables and Diagrams
Stoichiometry tables and diagrams are powerful tools to help you visualize and calculate the relationships between reactants and products. Here are some tips to use them effectively:
- Create a stoichiometry table to list the reactants, products, and their respective mole ratios.
- Use a diagram to show the relationships between the reactants and products.
- Identify the limiting reactant and highlight it in the table or diagram.
- Use the table or diagram to calculate the theoretical yield.
Example:
Suppose you have the following reaction:
A + 2B → C + D
You start with 1 mol of A and 2 mol of B. To calculate the theoretical yield of C, you would create a stoichiometry table and diagram:
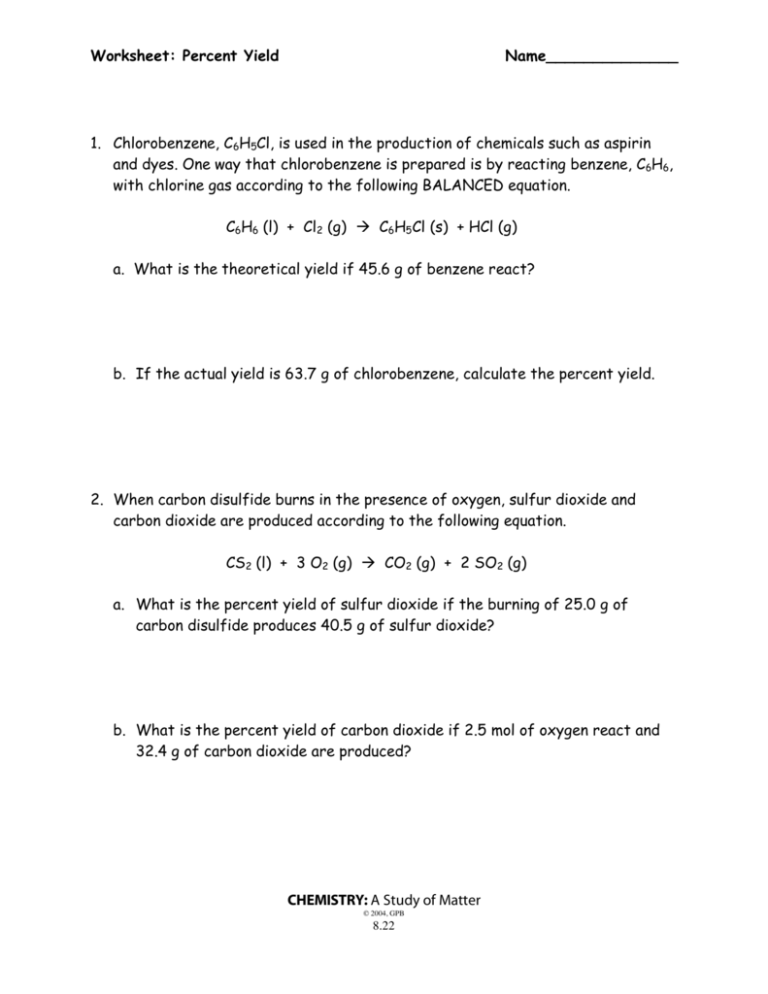
| Reactant | Product | Mole Ratio |
|---|---|---|
| A | C | 1:1 |
| B | C | 2:1 |
| B | D | 1:1 |
Diagram: A → C + D 2B → C + D
By using the table and diagram, you can easily identify the limiting reactant (A) and calculate the theoretical yield of C.
Additional Tips and Tricks
- Always use the correct units when calculating percent yield.
- Make sure to identify the limiting reactant correctly.
- Use a calculator to simplify calculations.
- Practice, practice, practice! The more you practice calculating percent yield, the more confident you will become.
And that’s it! By mastering these three methods, you will be able to calculate stoichiometry percent yield with ease. Remember to always use the correct units, identify the limiting reactant correctly, and practice, practice, practice!
What is the definition of percent yield?
+Percent yield is the ratio of the actual yield of a product to the theoretical yield, multiplied by 100%.
How do I calculate the theoretical yield?
+To calculate the theoretical yield, you need to know the balanced chemical equation, the number of moles of each reactant, and the molar mass of each product.
What is the purpose of a stoichiometry table and diagram?
+Stoichiometry tables and diagrams are powerful tools to help you visualize and calculate the relationships between reactants and products.
Related Terms:
- Stoichiometry percent yield Worksheet answers
- Percent yield Activity
- Percent yield questions
- Percentage yield pdf