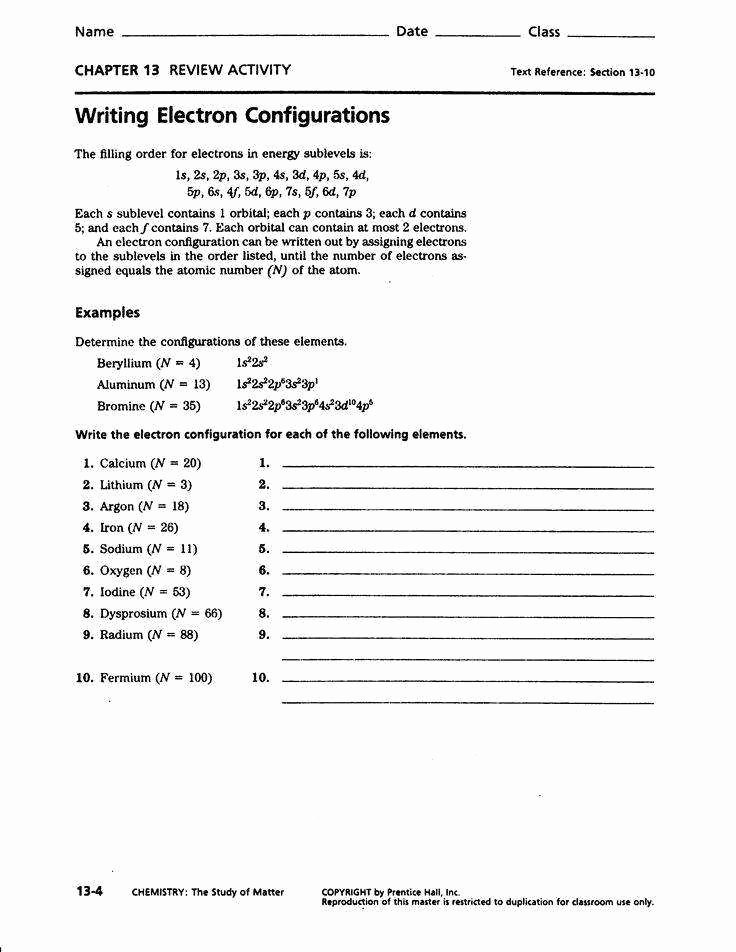
Electron Configuration Worksheet Answer Key Solutions
Understanding Electron Configuration: A Comprehensive Guide
Electron configuration is a fundamental concept in chemistry that describes the arrangement of electrons in an atom. It is a crucial aspect of understanding the periodic table and the behavior of elements. In this guide, we will delve into the world of electron configuration, exploring its principles, notation, and applications.
What is Electron Configuration?
Electron configuration refers to the arrangement of electrons in an atom, which is determined by the number of electrons and the energy levels they occupy. The electrons in an atom are arranged in a specific order, with each energy level accommodating a specific number of electrons. The energy levels are designated by integers (1, 2, 3, etc.), and each energy level has a specific capacity for electrons.
Aufbau Principle and Hund's Rule
The Aufbau principle states that electrons occupy the lowest available energy levels in an atom. This principle is fundamental in determining the electron configuration of an atom. Hund’s rule, on the other hand, states that when filling orbitals of equal energy, electrons occupy them singly and with parallel spins before pairing up.
Electron Configuration Notation
Electron configuration notation is a shorthand way of describing the arrangement of electrons in an atom. It consists of a series of numbers and letters that indicate the energy level, orbital type, and number of electrons. The notation is written in a specific format, with the energy level first, followed by the orbital type, and then the number of electrons.
Energy Levels and Orbitals
Energy levels, also known as electron shells, are the regions around the nucleus where electrons are found. Each energy level has a specific capacity for electrons, and the electrons in each energy level are arranged in orbitals. Orbitals are the regions within an energy level where electrons are most likely to be found.
s, p, d, and f Orbitals
There are four types of orbitals: s, p, d, and f. Each orbital type has a specific shape and can accommodate a specific number of electrons.
- s-orbitals are spherical and can accommodate up to 2 electrons.
- p-orbitals are dumbbell-shaped and can accommodate up to 6 electrons.
- d-orbitals are four-leaf clover-shaped and can accommodate up to 10 electrons.
- f-orbitals are six-lobed and can accommodate up to 14 electrons.
Electron Configuration Examples
Let’s examine the electron configuration of a few elements:
- Hydrogen (H): 1s1
- Helium (He): 1s2
- Oxygen (O): 1s2 2s2 2p4
- Nitrogen (N): 1s2 2s2 2p3
Electron Configuration Worksheet Answer Key Solutions
Here are the solutions to some electron configuration worksheet questions:
- What is the electron configuration of carbon ©?
- Answer: 1s2 2s2 2p2
- What is the electron configuration of neon (Ne)?
- Answer: 1s2 2s2 2p6
- What is the electron configuration of iron (Fe)?
- Answer: 1s2 2s2 2p6 3s2 3p6 3d6 4s2
Important Notes
📝 Note: Electron configuration is a fundamental concept in chemistry, and understanding it is crucial for understanding the periodic table and the behavior of elements.
Conclusion
In conclusion, electron configuration is a vital aspect of chemistry that describes the arrangement of electrons in an atom. Understanding the principles, notation, and applications of electron configuration is essential for grasping the behavior of elements and the periodic table. By mastering electron configuration, you will gain a deeper understanding of chemistry and be better equipped to tackle complex topics.
What is electron configuration?
+Electron configuration refers to the arrangement of electrons in an atom, which is determined by the number of electrons and the energy levels they occupy.
What is the Aufbau principle?
+The Aufbau principle states that electrons occupy the lowest available energy levels in an atom.
What is Hund’s rule?
+Hund’s rule states that when filling orbitals of equal energy, electrons occupy them singly and with parallel spins before pairing up.
Related Terms:
- Electron configuration worksheet pdf
- Electron configuration POGIL PDF
- Electron configuration pdf