7 Essential Atomic Structure Worksheet Answers Revealed

Unlocking the Secrets of Atomic Structure: A Comprehensive Guide
Atomic structure is a fundamental concept in chemistry and physics, and understanding it is crucial for students and professionals alike. In this article, we will delve into the world of atomic structure, exploring its key components, and providing answers to seven essential worksheet questions.
What is Atomic Structure?
Atomic structure refers to the arrangement of protons, neutrons, and electrons within an atom. It is the foundation of chemistry and physics, and understanding it is essential for grasping various chemical and physical phenomena.
Key Components of Atomic Structure
The atomic structure consists of three main components:
- Protons: Positively charged particles that reside in the nucleus of an atom.
- Neutrons: Particles with no charge that reside in the nucleus along with protons.
- Electrons: Negatively charged particles that orbit the nucleus.
Worksheet Question 1: What is the Atomic Number of an Element?
The atomic number of an element is the number of protons present in the nucleus of an atom. It defines the element’s identity and determines its position in the periodic table.
Worksheet Question 2: What is the Difference Between Protons and Neutrons?
Protons and neutrons are both found in the nucleus of an atom, but they differ in their charge. Protons are positively charged, while neutrons have no charge.
Worksheet Question 3: What is the Electron Configuration of an Atom?
The electron configuration of an atom is the arrangement of electrons in the atom’s energy levels or orbitals. It is typically written in a specific notation, with each energy level represented by a number and each orbital represented by a letter.
Worksheet Question 4: What is the Octet Rule?
The octet rule states that an atom tends to gain, lose, or share electrons to achieve a full outer energy level, which typically consists of eight electrons in the s and p orbitals.
Worksheet Question 5: What is the Difference Between Isotopes and Isobars?
Isotopes are atoms of the same element with different numbers of neutrons, while isobars are atoms of different elements with the same number of neutrons.
Worksheet Question 6: What is the Atomic Mass of an Element?
The atomic mass of an element is the average mass of its naturally occurring isotopes. It is typically expressed in units of atomic mass units (amu).
Worksheet Question 7: What is the Relationship Between Protons, Neutrons, and Electrons in an Atom?
The number of protons in an atom determines the number of electrons, which in turn determines the chemical properties of the element. The number of neutrons in an atom can vary, leading to the existence of isotopes.
🤔 Note: The number of protons, neutrons, and electrons in an atom is crucial for understanding its chemical and physical properties.
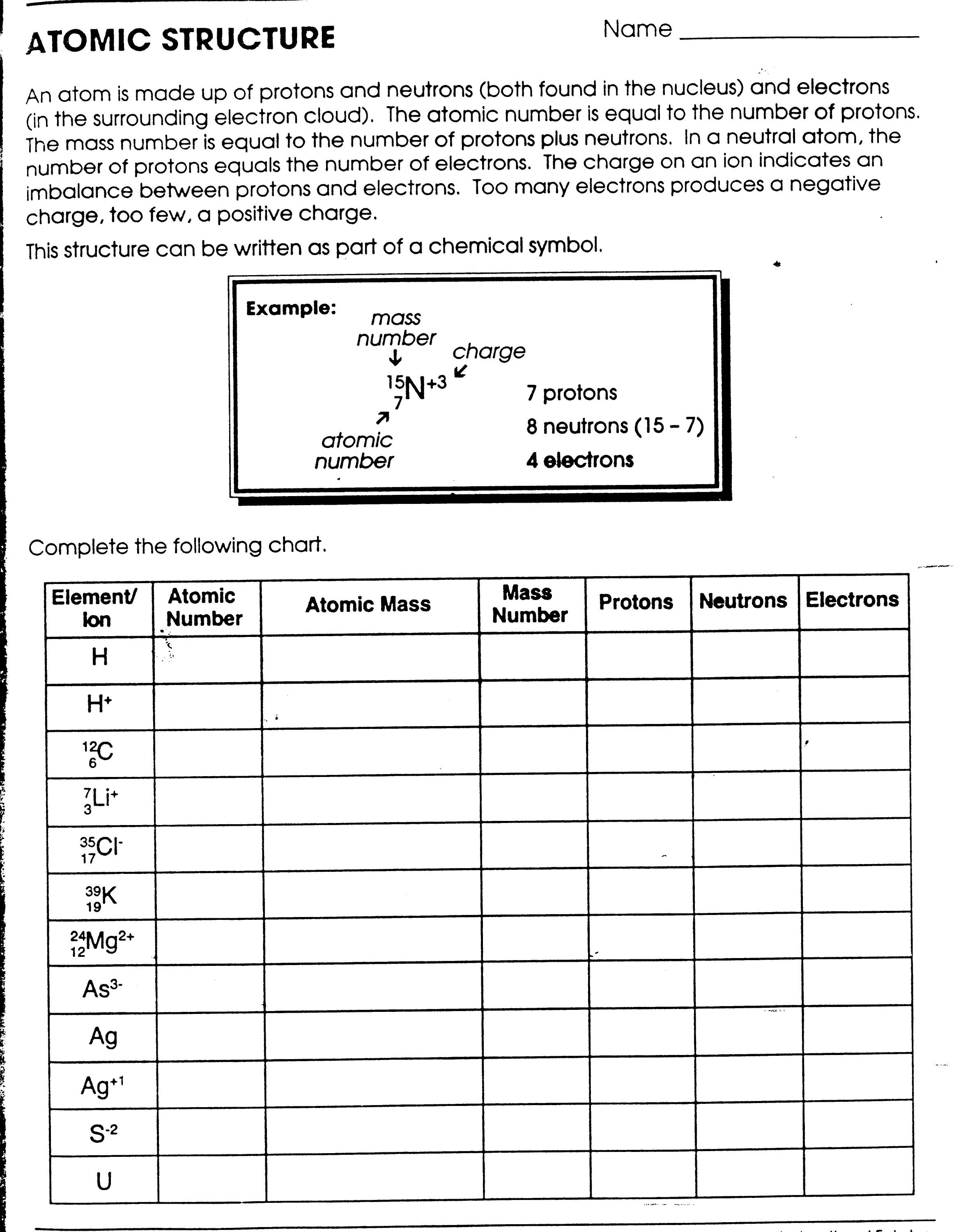
| Component | Charge | Location |
|---|---|---|
| Protons | Positive | Nucleus |
| Neutrons | No charge | Nucleus |
| Electrons | Negative | Orbitals |
By understanding the key components of atomic structure and their relationships, students and professionals can gain a deeper appreciation for the intricate world of chemistry and physics.
The intricacies of atomic structure are a fascinating topic, and mastering them can lead to a deeper understanding of various scientific concepts. By grasping the answers to these seven essential worksheet questions, individuals can build a strong foundation in chemistry and physics, paving the way for future discoveries and innovations.
What is the significance of atomic structure in chemistry and physics?
+Atomic structure is fundamental to understanding chemical and physical phenomena, including chemical reactions, thermodynamics, and quantum mechanics.
What is the difference between atomic number and atomic mass?
+Atomic number refers to the number of protons in an atom, while atomic mass is the average mass of an element’s naturally occurring isotopes.
How do electrons occupy energy levels in an atom?
+Electrons occupy energy levels in an atom according to the Aufbau principle and the Pauli exclusion principle, which dictate how electrons fill orbitals and energy levels.