Master Specific Heat with 5 Essential Calculation Tips
Understanding Specific Heat
Specific heat, also known as specific heat capacity, is the amount of heat energy required to raise the temperature of a unit mass of a substance by one degree Celsius (or Kelvin). It is a crucial concept in thermodynamics and is used to calculate the heat transfer between objects. In this article, we will explore the concept of specific heat and provide five essential calculation tips to help you master it.
Why is Specific Heat Important?
Specific heat is a vital concept in various fields, including physics, chemistry, and engineering. It helps us understand how different materials respond to temperature changes, which is essential in designing and optimizing systems, such as heating and cooling systems, engines, and refrigerators. Additionally, specific heat is used to calculate the energy required to heat or cool a substance, which is critical in various industrial and commercial applications.
What is the Formula for Specific Heat?
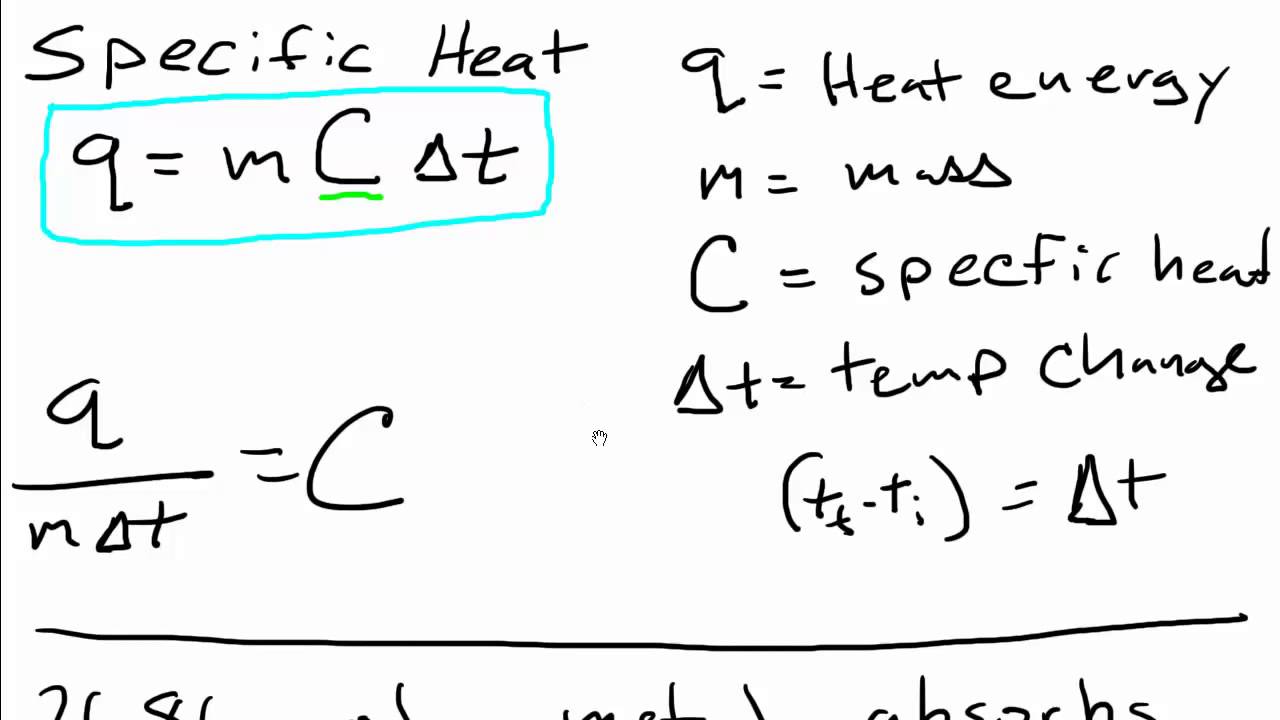
The formula for specific heat is:
Q = mcΔT
Where:
- Q is the amount of heat energy required (in joules, J)
- m is the mass of the substance (in kilograms, kg)
- c is the specific heat capacity (in joules per kilogram per degree Celsius, J/kg°C)
- ΔT is the change in temperature (in degree Celsius, °C)
5 Essential Calculation Tips for Specific Heat
Here are five essential calculation tips to help you master specific heat:
Tip 1: Understand the Units
When working with specific heat, it is essential to understand the units involved. The unit of specific heat is typically expressed in J/kg°C or J/g°C. Make sure to convert the units correctly to avoid errors in your calculations.
Tip 2: Use the Correct Formula
The formula for specific heat is Q = mcΔT. Make sure to use the correct formula and plug in the correct values to get the correct answer.
Tip 3: Pay Attention to the Sign of ΔT
The sign of ΔT is crucial in specific heat calculations. A positive ΔT indicates an increase in temperature, while a negative ΔT indicates a decrease in temperature. Make sure to pay attention to the sign of ΔT to avoid errors in your calculations.
Tip 4: Use the Correct Value of Specific Heat
The value of specific heat varies depending on the substance. Make sure to use the correct value of specific heat for the substance you are working with. You can find the specific heat values of common substances in tables or online resources.
Tip 5: Check Your Units and Answer
Finally, make sure to check your units and answer to ensure that they are correct. A simple mistake in units or calculation can lead to incorrect answers, so double-check your work to avoid errors.
📝 Note: Specific heat values can be found in tables or online resources. Make sure to use the correct value for the substance you are working with.
Example Calculation
Let’s say we want to calculate the amount of heat energy required to raise the temperature of 10 kg of water from 20°C to 80°C. The specific heat capacity of water is 4.184 J/g°C.
First, we need to convert the mass of water from kilograms to grams:
10 kg x 1000 g/kg = 10,000 g
Next, we can plug in the values into the formula:
Q = mcΔT Q = 10,000 g x 4.184 J/g°C x (80°C - 20°C) Q = 334,720 J
Therefore, the amount of heat energy required to raise the temperature of 10 kg of water from 20°C to 80°C is 334,720 J.
In Conclusion
Specific heat is a fundamental concept in thermodynamics, and mastering it requires practice and attention to detail. By following these five essential calculation tips, you can improve your understanding of specific heat and become proficient in calculating heat transfer between objects. Remember to always check your units and answer to ensure accuracy, and practice with different substances and temperature changes to reinforce your understanding.
What is specific heat capacity?
+Specific heat capacity is the amount of heat energy required to raise the temperature of a unit mass of a substance by one degree Celsius (or Kelvin).
What is the formula for specific heat?
+The formula for specific heat is Q = mcΔT, where Q is the amount of heat energy required, m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature.
What are some common substances with high specific heat capacity?
+Some common substances with high specific heat capacity include water, concrete, and brick.