5 Ways to Master the Solubility Curve Worksheet
Unlocking the Secrets of Solubility: Mastering the Solubility Curve Worksheet
The solubility curve worksheet is a fundamental tool in chemistry that helps students understand the relationship between temperature and solubility. It’s a graphical representation of how the solubility of a substance changes as the temperature increases or decreases. Mastering the solubility curve worksheet is essential for any chemistry student, as it allows them to predict the solubility of a substance at different temperatures. In this article, we’ll explore five ways to master the solubility curve worksheet and take your chemistry skills to the next level.
1. Understand the Basics of Solubility
Before diving into the solubility curve worksheet, it’s essential to understand the basics of solubility. Solubility is the maximum amount of a substance that can dissolve in a given amount of solvent at a particular temperature. The solubility curve worksheet typically plots the solubility of a substance against temperature.
Key terms to remember:
- Solubility: The maximum amount of a substance that can dissolve in a given amount of solvent at a particular temperature.
- Solvent: The substance in which the solute dissolves.
- Solute: The substance that dissolves in the solvent.
2. Learn to Read the Solubility Curve
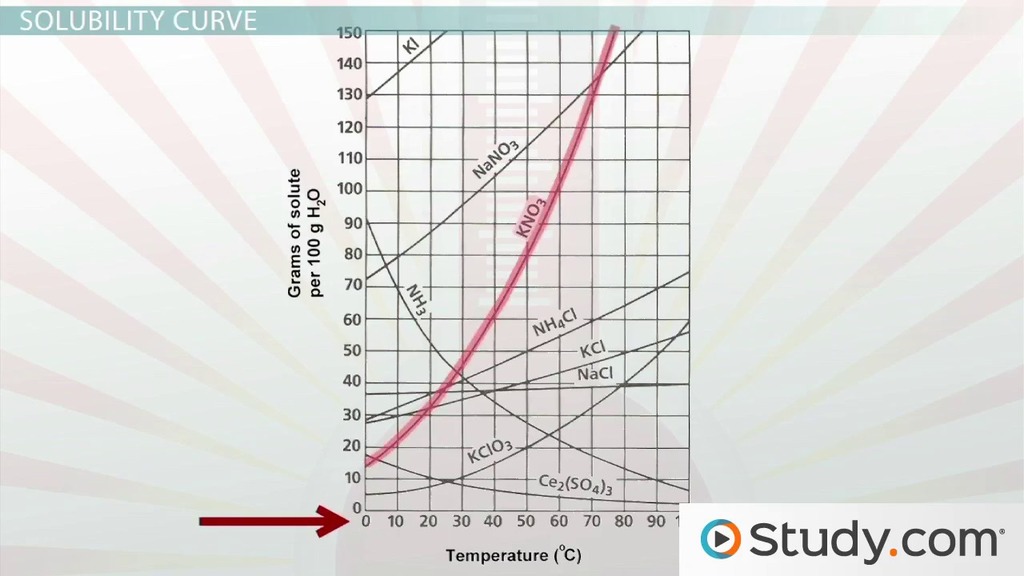
Reading the solubility curve worksheet requires understanding the different parts of the graph.
- X-axis: Represents the temperature in degrees Celsius (°C) or Kelvin (K).
- Y-axis: Represents the solubility of the substance in grams per 100 grams of solvent (g/100g).
- Curve: The line that plots the solubility of the substance against temperature.
Important notes:
- A positive slope indicates that the solubility increases as the temperature increases.
- A negative slope indicates that the solubility decreases as the temperature increases.
- A horizontal line indicates that the solubility remains constant over a range of temperatures.
💡 Note: When reading the solubility curve, make sure to pay attention to the units on the x and y axes, as they can affect your interpretation of the data.
3. Practice Plotting Solubility Curves
Plotting solubility curves is an essential skill for mastering the solubility curve worksheet. Practice plotting different types of solubility curves, including:
- Straight lines: Indicate a constant solubility over a range of temperatures.
- Curves with a positive slope: Indicate an increase in solubility as temperature increases.
- Curves with a negative slope: Indicate a decrease in solubility as temperature increases.
Tips for plotting solubility curves:
- Use a ruler to draw a straight line or a smooth curve.
- Make sure to label the x and y axes correctly.
- Use a pencil to plot the curve, as it’s easier to erase mistakes.
4. Analyze and Interpret Solubility Curves
Analyzing and interpreting solubility curves is a critical skill for mastering the solubility curve worksheet. Practice answering questions like:
- What is the solubility of the substance at a given temperature?
- How does the solubility of the substance change as the temperature increases or decreases?
- What is the maximum solubility of the substance?
Tips for analyzing and interpreting solubility curves:
- Use the solubility curve to identify the maximum solubility of the substance.
- Analyze the slope of the curve to determine how the solubility changes as the temperature increases or decreases.
- Use the solubility curve to predict the solubility of the substance at different temperatures.
5. Apply Solubility Curves to Real-World Problems
Applying solubility curves to real-world problems is an essential skill for mastering the solubility curve worksheet. Practice solving problems like:
- Designing a crystallization process: Use the solubility curve to determine the optimal temperature for crystallization.
- Predicting the solubility of a substance: Use the solubility curve to predict the solubility of a substance at a given temperature.
- Optimizing a chemical reaction: Use the solubility curve to determine the optimal temperature for a chemical reaction.
Tips for applying solubility curves to real-world problems:
- Use the solubility curve to identify the optimal temperature for a process or reaction.
- Analyze the solubility curve to predict the solubility of a substance at different temperatures.
- Use the solubility curve to optimize a process or reaction.
What is the solubility curve worksheet?
+The solubility curve worksheet is a graphical representation of the relationship between temperature and solubility. It plots the solubility of a substance against temperature, allowing students to predict the solubility of a substance at different temperatures.
How do I read the solubility curve?
+To read the solubility curve, pay attention to the x and y axes, which represent temperature and solubility, respectively. A positive slope indicates an increase in solubility as temperature increases, while a negative slope indicates a decrease in solubility as temperature increases.
What is the importance of mastering the solubility curve worksheet?
+Mastering the solubility curve worksheet is essential for predicting the solubility of a substance at different temperatures, which is crucial in various chemical processes and reactions. It also helps students understand the relationship between temperature and solubility, which is a fundamental concept in chemistry.
By following these five ways to master the solubility curve worksheet, you’ll be able to unlock the secrets of solubility and take your chemistry skills to the next level. Remember to practice plotting and analyzing solubility curves, and apply them to real-world problems to become a master of solubility.