Understanding Atoms: Protons Electrons and Neutrons Worksheet
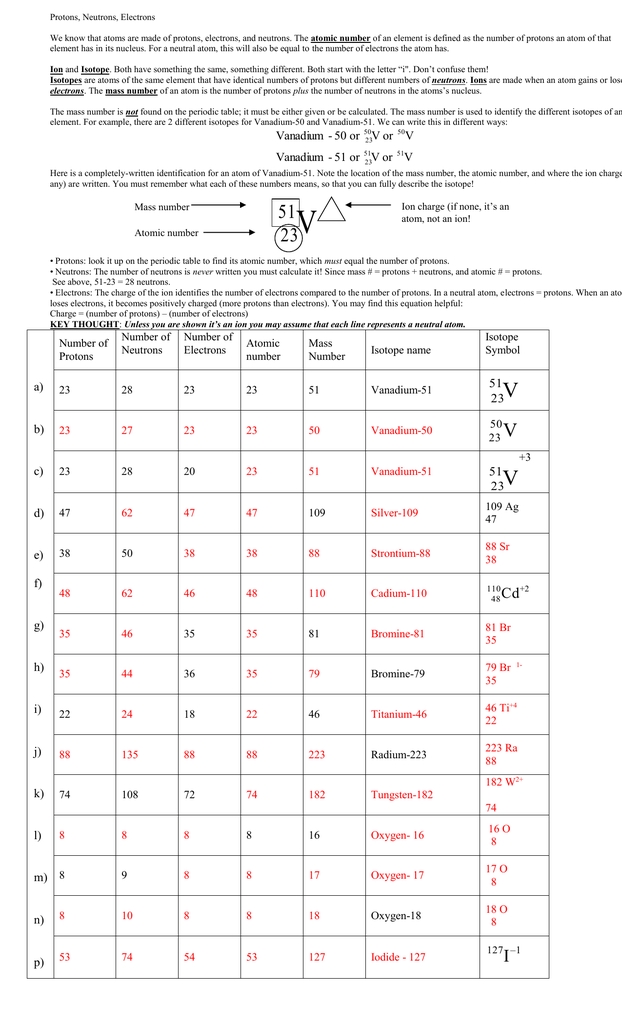
Unlocking the Secrets of Atoms: A Comprehensive Guide to Protons, Electrons, and Neutrons
Atoms are the fundamental building blocks of matter, and understanding their composition is crucial for grasping various scientific concepts. This blog post will delve into the world of atoms, exploring the roles of protons, electrons, and neutrons. We will also provide a worksheet to help reinforce your understanding of these atomic components.
What are Atoms?
Atoms are the smallest units of a chemical element, and they consist of three primary particles: protons, electrons, and neutrons. Atoms are the foundation of matter, and they cannot be created or destroyed in chemical reactions. Instead, they are rearranged to form new substances.
Protons: The Positive Force
Protons are positively charged particles that reside in the nucleus of an atom. The number of protons in an atom determines the element of an atom, and each element has a unique number of protons in its atoms. For example, hydrogen atoms have one proton, while oxygen atoms have eight protons.
👍 Note: The number of protons in an atom is also known as the atomic number.
Neutrons: The Neutral Component
Neutrons are particles that have no charge and are found in the nucleus along with protons. The number of neutrons in an atom can vary, leading to different isotopes of the same element. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons.
Electrons: The Negatively Charged Particles
Electrons are negatively charged particles that orbit the nucleus of an atom. The number of electrons in an atom is equal to the number of protons, and this number determines the chemical properties of an element. Electrons are arranged in energy levels or shells around the nucleus, and each shell has a specific capacity for electrons.
Atomic Structure: A Closer Look
The atomic structure consists of the nucleus, which contains protons and neutrons, and the electron cloud, which is the region around the nucleus where electrons are found. The nucleus is incredibly small compared to the size of the atom, and the electrons are in constant motion, creating a cloud-like structure around the nucleus.
Worksheet: Understanding Atoms
Section 1: Multiple Choice Questions
- What is the smallest unit of a chemical element? a) Molecule b) Atom c) Compound d) Mixture
Answer: b) Atom
- What determines the element of an atom? a) Number of electrons b) Number of protons c) Number of neutrons d) Number of energy levels
Answer: b) Number of protons
Section 2: Short Answer Questions
- Describe the role of protons in an atom.
Answer: Protons are positively charged particles that reside in the nucleus of an atom and determine the element of an atom.
- What is the difference between isotopes and elements?
Answer: Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. Elements are atoms with the same number of protons.
Section 3: Fill-in-the-Blank Questions
- The number of protons in an atom is also known as the _______________________ number.
Answer: atomic
- Neutrons are particles that have no _______________________.
Answer: charge
Section 4: True or False Questions
- True or False: Atoms can be created or destroyed in chemical reactions.
Answer: False
- True or False: The number of electrons in an atom is always equal to the number of protons.
Answer: True
Atomic Worksheet Answer Key
Section 1: Multiple Choice Questions
- b) Atom
- b) Number of protons
Section 2: Short Answer Questions
- Protons are positively charged particles that reside in the nucleus of an atom and determine the element of an atom.
- Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. Elements are atoms with the same number of protons.
Section 3: Fill-in-the-Blank Questions
- atomic
- charge
Section 4: True or False Questions
- False
- True
By completing this worksheet, you have taken the first step in understanding the fascinating world of atoms. Remember, atoms are the building blocks of matter, and grasping their composition is essential for understanding various scientific concepts.
What is the difference between an atom and a molecule?
+An atom is the smallest unit of a chemical element, while a molecule is a group of atoms bonded together.
What determines the chemical properties of an element?
+The number of electrons in an atom determines the chemical properties of an element.
What is the role of neutrons in an atom?
+Neutrons are particles that have no charge and are found in the nucleus along with protons. They help determine the mass of an atom and can vary in number, leading to different isotopes of the same element.