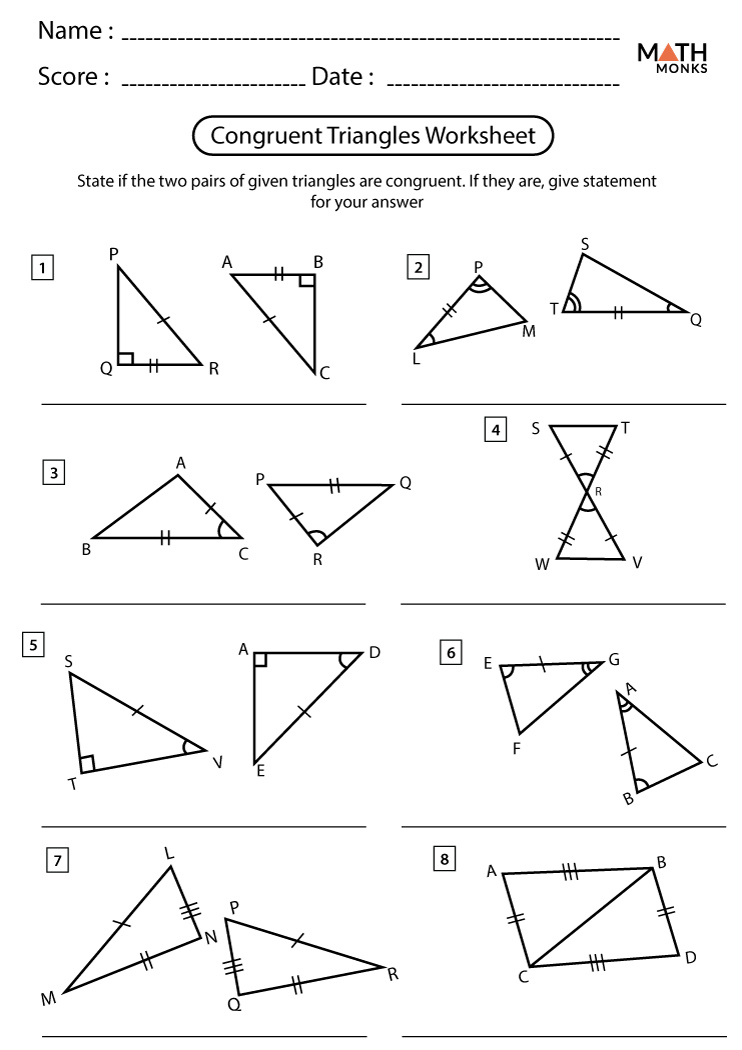
Mastering Solubility Curves: Practice Worksheet

Mastering Solubility Curves: Practice Worksheet
Understanding solubility curves is crucial in chemistry, particularly when dealing with the behavior of substances in different temperatures and solvents. A solubility curve is a graphical representation of the solubility of a substance in a solvent at various temperatures. In this practice worksheet, we will delve into the world of solubility curves, exploring their interpretation, applications, and practice problems to reinforce your understanding.
Interpreting Solubility Curves
A solubility curve is typically plotted with temperature on the x-axis and solubility (in grams per 100 mL of solvent) on the y-axis. The curve shows the maximum amount of a substance that can dissolve in a given amount of solvent at a particular temperature. Here are some key points to remember when interpreting solubility curves:
- Increasing temperature: For most substances, solubility increases with increasing temperature. This means that as the temperature rises, more of the substance can dissolve in the same amount of solvent.
- Decreasing temperature: Conversely, as the temperature decreases, the solubility of most substances decreases, meaning less of the substance can dissolve in the same amount of solvent.
- Solubility maximum: Some substances exhibit a maximum solubility at a specific temperature. Beyond this point, increasing the temperature can actually decrease the solubility.
📝 Note: Understanding the shape of a solubility curve is crucial for predicting the behavior of substances in different conditions.
Applications of Solubility Curves
Solubility curves have numerous practical applications in various fields, including:
- Chemical engineering: Solubility curves are used to design and optimize processes involving the dissolution of substances.
- Pharmaceuticals: Understanding the solubility of drugs is crucial for determining their efficacy and stability.
- Food science: Solubility curves are used to predict the behavior of food ingredients and additives in different conditions.
Practice Problems
Here are some practice problems to help you reinforce your understanding of solubility curves:
Problem 1
Given the solubility curve below, determine the maximum amount of substance A that can dissolve in 100 mL of water at 25°C.

| Temperature (°C) | Solubility (g/100 mL) |
|---|---|
| 10 | 5 |
| 20 | 10 |
| 25 | 15 |
| 30 | 20 |
Problem 2
A substance B has a solubility curve that shows a maximum solubility of 30 g/100 mL at 40°C. If the temperature is increased to 50°C, what happens to the solubility of substance B?
Problem 3
Given the solubility curve below, determine the temperature at which the solubility of substance C is 25 g/100 mL.
| Temperature (°C) | Solubility (g/100 mL) |
|---|---|
| 15 | 10 |
| 25 | 20 |
| 35 | 30 |
| 45 | 25 |
📝 Note: Take your time to work through these practice problems and check your answers against the solutions provided below.
Solutions
Solution 1
From the solubility curve, we can see that the maximum amount of substance A that can dissolve in 100 mL of water at 25°C is 15 g.
Solution 2
Since the temperature is increased beyond the maximum solubility point, the solubility of substance B decreases. Therefore, the solubility of substance B at 50°C is less than 30 g/100 mL.
Solution 3
From the solubility curve, we can see that the temperature at which the solubility of substance C is 25 g/100 mL is 45°C.
In conclusion, mastering solubility curves is essential for predicting the behavior of substances in different conditions. By understanding the interpretation and applications of solubility curves, you can better navigate the world of chemistry and make informed decisions in various fields.
What is a solubility curve?
+A solubility curve is a graphical representation of the solubility of a substance in a solvent at various temperatures.
How does temperature affect solubility?
+For most substances, solubility increases with increasing temperature. However, some substances exhibit a maximum solubility at a specific temperature.
What are some practical applications of solubility curves?
+Solubility curves have numerous practical applications in various fields, including chemical engineering, pharmaceuticals, and food science.
Related Terms:
- Kalium sulfat
- Kalium klorida
- Air
- Solubility curve Worksheet with Answers
- Solubility curve questions and answers
- Solubility Worksheet PDF