5 Ways to Master Phase Change Worksheet Answers

Unlocking the Secrets of Phase Change: A Comprehensive Guide
Phase change is a fundamental concept in physics and chemistry that involves the transformation of a substance from one state of matter to another. Mastering phase change worksheets is essential for students to grasp the underlying principles and apply them to real-world problems. In this article, we will explore five ways to master phase change worksheet answers and provide a comprehensive guide to help you excel in this area.
1. Understanding the Basics of Phase Change
Before diving into phase change worksheets, it is crucial to understand the basics of phase change. Phase change occurs when a substance changes its state of matter, such as from solid to liquid or from liquid to gas. There are several types of phase changes, including:
- Melting: the change from solid to liquid
- Boiling: the change from liquid to gas
- Condensation: the change from gas to liquid
- Sublimation: the change from solid to gas
- Deposition: the change from gas to solid
Each type of phase change has its own unique characteristics and requirements.
2. Familiarizing Yourself with Phase Change Diagrams
Phase change diagrams are a crucial tool for visualizing and understanding phase change. These diagrams show the relationship between temperature, pressure, and the state of matter of a substance. By studying phase change diagrams, you can gain insight into the conditions under which phase change occurs and how it affects the properties of a substance.
Here is an example of a phase change diagram:
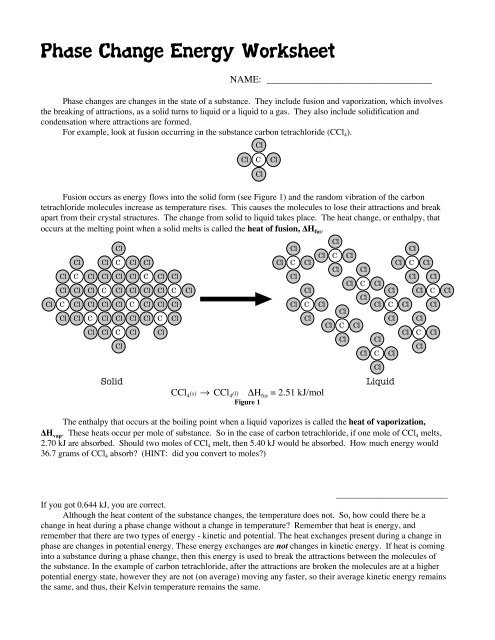
| Temperature (°C) | Pressure (atm) | State of Matter |
|---|---|---|
| 0 | 1 | Solid |
| 100 | 1 | Liquid |
| 100 | 0.5 | Gas |
3. Practicing Phase Change Problems
Practice is key to mastering phase change worksheet answers. By working through practice problems, you can develop your problem-solving skills and become more comfortable with the concepts and terminology associated with phase change.
Here are some examples of phase change problems:
- What is the melting point of ice at standard pressure?
- What is the boiling point of water at 2 atm?
- What is the state of matter of carbon dioxide at -78.5°C and 1 atm?
4. Using Online Resources and Study Guides
There are many online resources and study guides available to help you master phase change worksheet answers. These resources can provide additional practice problems, tutorials, and explanations to supplement your textbook and classroom instruction.
Some popular online resources include:
- Khan Academy
- Crash Course
- Physics Classroom
5. Reviewing and Reflecting on Your Work
Finally, it is essential to review and reflect on your work to master phase change worksheet answers. By reviewing your mistakes and reflecting on what you have learned, you can identify areas for improvement and solidify your understanding of phase change concepts.
Here are some tips for reviewing and reflecting on your work:
- Review your mistakes and try to understand where you went wrong
- Reflect on what you have learned and how you can apply it to real-world problems
- Use flashcards or concept maps to reinforce your understanding of key terms and concepts
💡 Note: Mastering phase change worksheet answers requires practice, patience, and persistence. Don't be afraid to ask for help or seek additional resources if you need them.
In conclusion, mastering phase change worksheet answers requires a combination of understanding the basics of phase change, familiarizing yourself with phase change diagrams, practicing phase change problems, using online resources and study guides, and reviewing and reflecting on your work. By following these steps and staying committed to your studies, you can excel in phase change and achieve academic success.
What is the difference between melting and boiling?
+Melting is the change from solid to liquid, while boiling is the change from liquid to gas.
What is the state of matter of water at 0°C and 1 atm?
+At 0°C and 1 atm, water is in its solid state (ice).
What is the boiling point of water at standard pressure?
+The boiling point of water at standard pressure is 100°C.