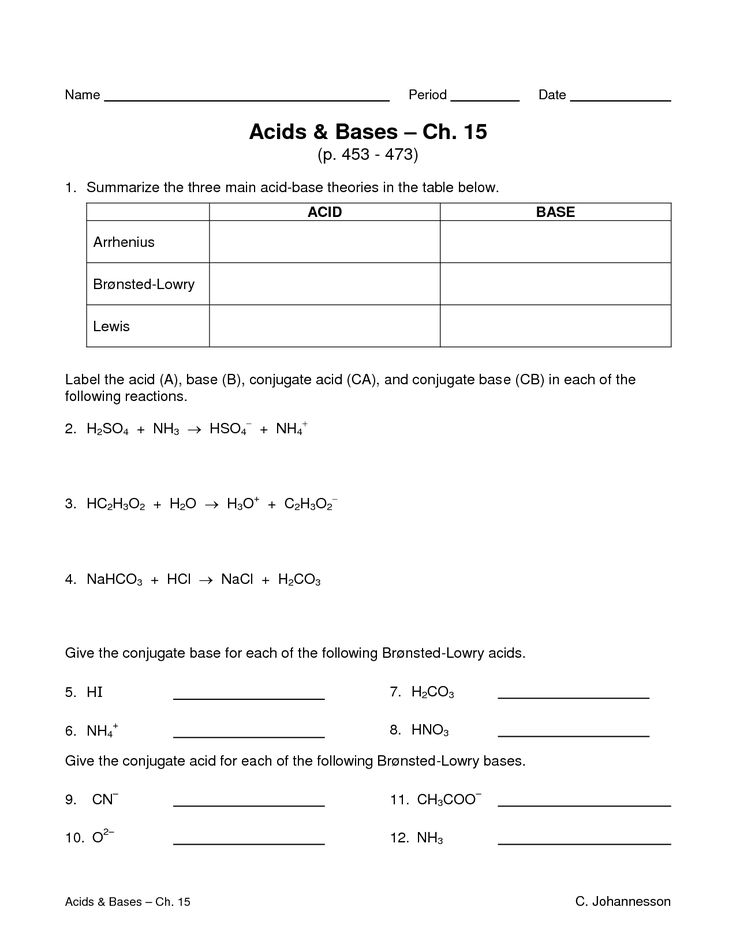
PH and pOH Worksheet for Acid-Base Chemistry Mastery
Understanding pH and pOH: A Comprehensive Guide to Acid-Base Chemistry
Acid-base chemistry is a fundamental concept in chemistry, and understanding pH and pOH is crucial for mastering this topic. In this worksheet, we will delve into the world of pH and pOH, exploring their definitions, calculations, and applications.
What is pH?
pH is a measure of the concentration of hydrogen ions (H+) in a solution. It is defined as the negative logarithm of the hydrogen ion concentration:
pH = -log[H+]
A pH of 7 is neutral, while a pH less than 7 is acidic and a pH greater than 7 is basic.
What is pOH?
pOH is a measure of the concentration of hydroxide ions (OH-) in a solution. It is defined as the negative logarithm of the hydroxide ion concentration:
pOH = -log[OH-]
A pOH of 7 is neutral, while a pOH less than 7 is basic and a pOH greater than 7 is acidic.
The Relationship Between pH and pOH
pH and pOH are related by the following equation:
pH + pOH = 14
This equation shows that as the pH of a solution increases, the pOH decreases, and vice versa.
Calculating pH and pOH
To calculate pH and pOH, you need to know the concentration of hydrogen ions (H+) and hydroxide ions (OH-) in the solution. The following steps outline the calculation process:
- Determine the concentration of hydrogen ions (H+) or hydroxide ions (OH-) in the solution.
- Use the formula pH = -log[H+] or pOH = -log[OH-] to calculate the pH or pOH.
- Use the equation pH + pOH = 14 to find the other value.
Example:
A solution has a hydrogen ion concentration of 0.0001 M. Calculate the pH and pOH.
pH = -log(0.0001) = 4 pOH = 14 - pH = 14 - 4 = 10
Applications of pH and pOH
pH and pOH have numerous applications in various fields, including:
- Chemical industry: pH and pOH are used to monitor and control the acidity and basicity of chemical reactions.
- Environmental science: pH and pOH are used to measure the acidity and basicity of water and soil.
- Biology: pH and pOH are used to understand the acidity and basicity of biological systems, such as the human body.
- Food industry: pH and pOH are used to control the acidity and basicity of food products.
pH and pOH Worksheet
Complete the following problems to practice calculating pH and pOH:

| Concentration of H+ or OH- | pH | pOH |
|---|---|---|
| 0.01 M H+ | ||
| 0.001 M OH- | ||
| 0.0001 M H+ | ||
| 0.1 M OH- |
Answers:
| Concentration of H+ or OH- | pH | pOH |
|---|---|---|
| 0.01 M H+ | 2 | 12 |
| 0.001 M OH- | 3 | |
| 0.0001 M H+ | 4 | 10 |
| 0.1 M OH- | 1 |
💡 Note: Use the formulas pH = -log[H+] and pOH = -log[OH-] to calculate the pH and pOH values.
What is the difference between pH and pOH?
+pH measures the concentration of hydrogen ions (H+) in a solution, while pOH measures the concentration of hydroxide ions (OH-).
How are pH and pOH related?
+pH and pOH are related by the equation pH + pOH = 14.
What is the significance of pH and pOH in real-life applications?
+pH and pOH have numerous applications in various fields, including chemical industry, environmental science, biology, and food industry.
Mastering pH and pOH is essential for understanding acid-base chemistry. By practicing the calculations and understanding the applications, you can develop a deeper appreciation for the importance of pH and pOH in various fields.