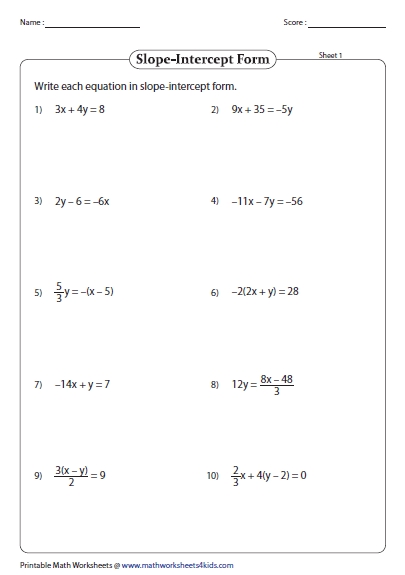
Periodic Trends Practice Worksheet

Understanding Periodic Trends
Periodic trends are patterns that occur in the periodic table, which can be used to predict the properties and behavior of elements. In this practice worksheet, we will explore some of the key periodic trends and how they can be used to make predictions.
Atomic Radius
The atomic radius is the distance from the nucleus of an atom to the outermost electron. As we move from left to right across a period, the atomic radius generally decreases. This is because the number of protons in the nucleus increases, which pulls the electrons closer to the nucleus.
| Element | Atomic Radius (pm) |
|---|---|
| Lithium (Li) | 152 |
| Beryllium (Be) | 112 |
| Boron (B) | 87 |
| Carbon © | 67 |
| Nitrogen (N) | 56 |
| Oxygen (O) | 48 |
| Fluorine (F) | 42 |
| Neon (Ne) | 38 |
💡 Note: pm stands for picometers, which is a unit of length equal to one-trillionth of a meter.
Questions:
- Which element has the largest atomic radius in the table above?
- Which element has the smallest atomic radius in the table above?
- As we move from left to right across a period, what happens to the atomic radius?
Electronegativity
Electronegativity is a measure of an atom’s ability to attract electrons. As we move from left to right across a period, the electronegativity generally increases. This is because the number of protons in the nucleus increases, which pulls the electrons closer to the nucleus and increases the atom’s ability to attract electrons.
| Element | Electronegativity |
|---|---|
| Lithium (Li) | 0.98 |
| Beryllium (Be) | 1.57 |
| Boron (B) | 2.04 |
| Carbon © | 2.55 |
| Nitrogen (N) | 3.04 |
| Oxygen (O) | 3.44 |
| Fluorine (F) | 3.98 |
| Neon (Ne) | 4.79 |
💡 Note: Electronegativity values are relative and can vary depending on the specific scale used.
Questions:
- Which element has the highest electronegativity value in the table above?
- Which element has the lowest electronegativity value in the table above?
- As we move from left to right across a period, what happens to the electronegativity?
Ionization Energy
Ionization energy is the energy required to remove an electron from an atom. As we move from left to right across a period, the ionization energy generally increases. This is because the number of protons in the nucleus increases, which pulls the electrons closer to the nucleus and makes it more difficult to remove an electron.
| Element | Ionization Energy (kJ/mol) |
|---|---|
| Lithium (Li) | 520 |
| Beryllium (Be) | 900 |
| Boron (B) | 801 |
| Carbon © | 1086 |
| Nitrogen (N) | 1402 |
| Oxygen (O) | 1314 |
| Fluorine (F) | 1681 |
| Neon (Ne) | 2081 |
💡 Note: Ionization energy values are typically measured in kJ/mol (kilojoules per mole).
Questions:
- Which element has the lowest ionization energy value in the table above?
- Which element has the highest ionization energy value in the table above?
- As we move from left to right across a period, what happens to the ionization energy?
Answers:
- Lithium (Li)
- Neon (Ne)
- The atomic radius decreases, the electronegativity increases, and the ionization energy increases.
Conclusion:
In this practice worksheet, we explored some of the key periodic trends and how they can be used to make predictions. By understanding these trends, we can better understand the properties and behavior of elements and make more informed decisions in fields such as chemistry and physics.
FAQ Section:
What is the periodic table?
+The periodic table is a tabular arrangement of the known chemical elements, organized by their atomic number (number of protons in the nucleus), electron configuration, and recurring chemical properties.
What is electronegativity?
+Electronegativity is a measure of an atom’s ability to attract electrons in a covalent bond.
What is ionization energy?
+Ionization energy is the energy required to remove an electron from an atom.
Related Terms:
- Ikatan kimia
- Tabel Periodik
- Atom
- Hidrogen
- Tren periodik
- Periodic Trends Questions and Answers