Mastering the Periodic Table Basics Worksheet
Unlocking the Secrets of the Periodic Table: A Comprehensive Guide
The periodic table is a fundamental tool in chemistry, providing a visual representation of the elements and their relationships. Mastering the basics of the periodic table is essential for understanding chemistry and its applications. In this article, we will delve into the world of the periodic table, exploring its history, structure, and key concepts.
A Brief History of the Periodic Table
The periodic table has undergone significant transformations since its inception. The concept of a periodic table was first introduced by Dmitri Mendeleev in 1869. Mendeleev, a Russian chemist, recognized that elements with similar properties recurred at regular intervals when listed in order of atomic weight. He developed a table that organized elements into rows (periods) and columns (groups) based on their properties.
Over time, the periodic table has evolved to include new elements, revisions to the existing structure, and the addition of new rows and columns. Today, the periodic table is a powerful tool that helps chemists and scientists understand the relationships between elements and their properties.
Understanding the Structure of the Periodic Table
The periodic table consists of rows (periods) and columns (groups). The elements are arranged in order of increasing atomic number (number of protons in the nucleus) from left to right and top to bottom.
- Periods: The horizontal rows of the periodic table are called periods. Elements in the same period have the same number of electron shells.
- Groups: The vertical columns of the periodic table are called groups or families. Elements in the same group have similar chemical properties due to the same number of electrons in their outermost energy level.
- Blocks: The periodic table can also be divided into blocks, which are determined by the orbital type of the outermost electrons. The blocks are s, p, d, and f.
Key Concepts: Metals, Nonmetals, and Metalloids
The periodic table is often divided into three main categories: metals, nonmetals, and metalloids.
- Metals: Metals are typically found on the left side and in the center of the periodic table. They are usually shiny, malleable, and good conductors of electricity.
- Nonmetals: Nonmetals are typically found on the right side of the periodic table. They are often dull, brittle, and poor conductors of electricity.
- Metalloids: Metalloids are found on the border between metals and nonmetals. They exhibit some properties of metals and some properties of nonmetals.
The Periodic Table Families
The periodic table is composed of several families or groups of elements with similar properties. Some of the most notable families include:
- Alkali Metals (Group 1): Highly reactive metals that readily lose one electron to form a positive ion.
- Noble Gases (Group 18): Unreactive gases that have a full outer energy level.
- Halogens (Group 17): Highly reactive nonmetals that readily gain one electron to form a negative ion.
Valence Electrons and the Periodic Table
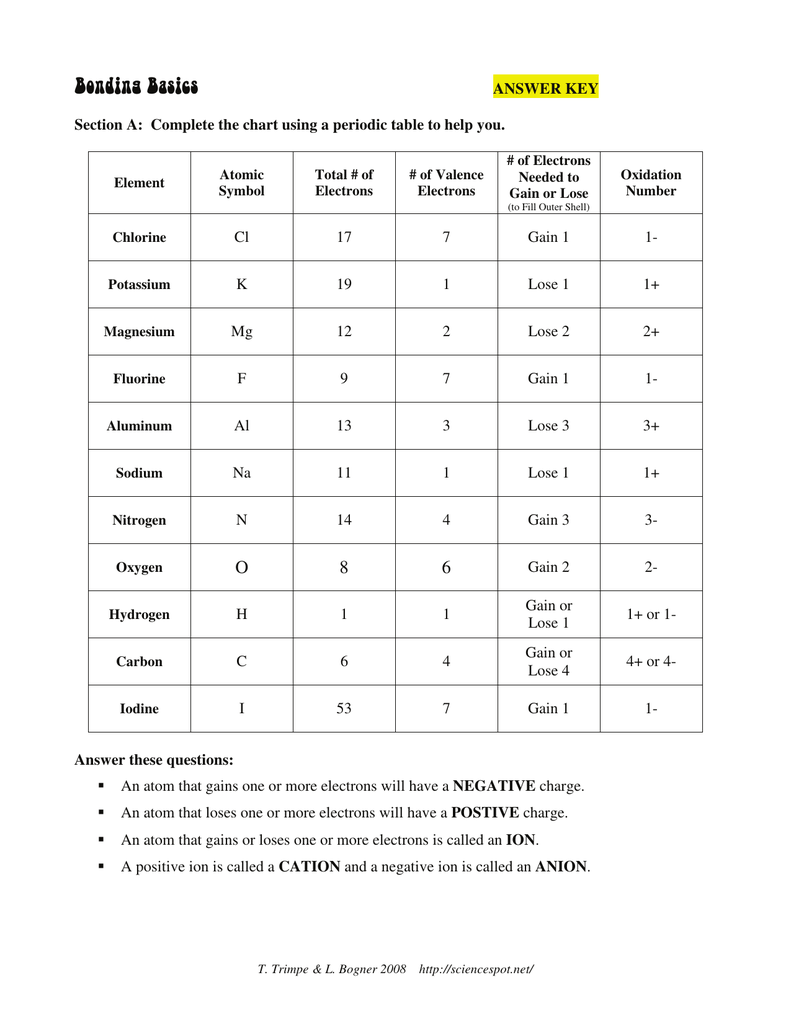
Valence electrons are the electrons in the outermost energy level of an atom. The number of valence electrons an element has determines its chemical properties and its position in the periodic table.
- Valence Electrons and Reactivity: Elements with a full outer energy level (noble gases) are unreactive, while elements with a nearly full outer energy level (halogens) are highly reactive.
- Valence Electrons and Ion Formation: Elements with a few valence electrons (alkali metals) readily lose electrons to form positive ions, while elements with many valence electrons (halogens) readily gain electrons to form negative ions.
💡 Note: The periodic table is a powerful tool that helps us understand the relationships between elements and their properties. By mastering the basics of the periodic table, we can gain a deeper understanding of chemistry and its applications.
Applying the Periodic Table to Real-World Scenarios
The periodic table has numerous applications in various fields, including chemistry, physics, and materials science.
- Predicting Chemical Reactivity: The periodic table can be used to predict how elements will react with each other.
- Identifying Element Properties: The periodic table can be used to identify the properties of an element, such as its melting point, boiling point, and conductivity.
Conclusion
The periodic table is a fundamental tool in chemistry that provides a visual representation of the elements and their relationships. By mastering the basics of the periodic table, we can gain a deeper understanding of chemistry and its applications. The periodic table has numerous applications in various fields, including chemistry, physics, and materials science.
What is the periodic table?
+The periodic table is a tabular arrangement of the elements, organized by their atomic number, electron configuration, and recurring chemical properties.
What are the main categories of elements in the periodic table?
+The main categories of elements in the periodic table are metals, nonmetals, and metalloids.
What is the significance of valence electrons in the periodic table?
+Valence electrons determine the chemical properties of an element and its position in the periodic table. They also play a crucial role in the formation of ions and chemical compounds.
Related Terms:
- Hidrogen
- Atom
- Kimia
- Logam
- Tabel Periodik
- Periodic table Basics pdf