10 Essential Parts of the Atom You Should Know
Understanding the Atom: A Comprehensive Guide
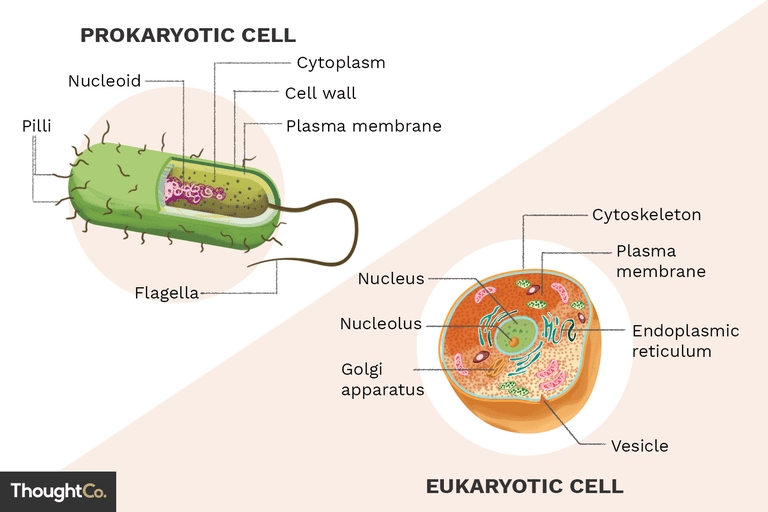
The atom is the building block of matter, and it’s essential to understand its components to grasp the fundamental principles of chemistry and physics. In this article, we’ll delve into the 10 essential parts of the atom you should know.
1. Protons
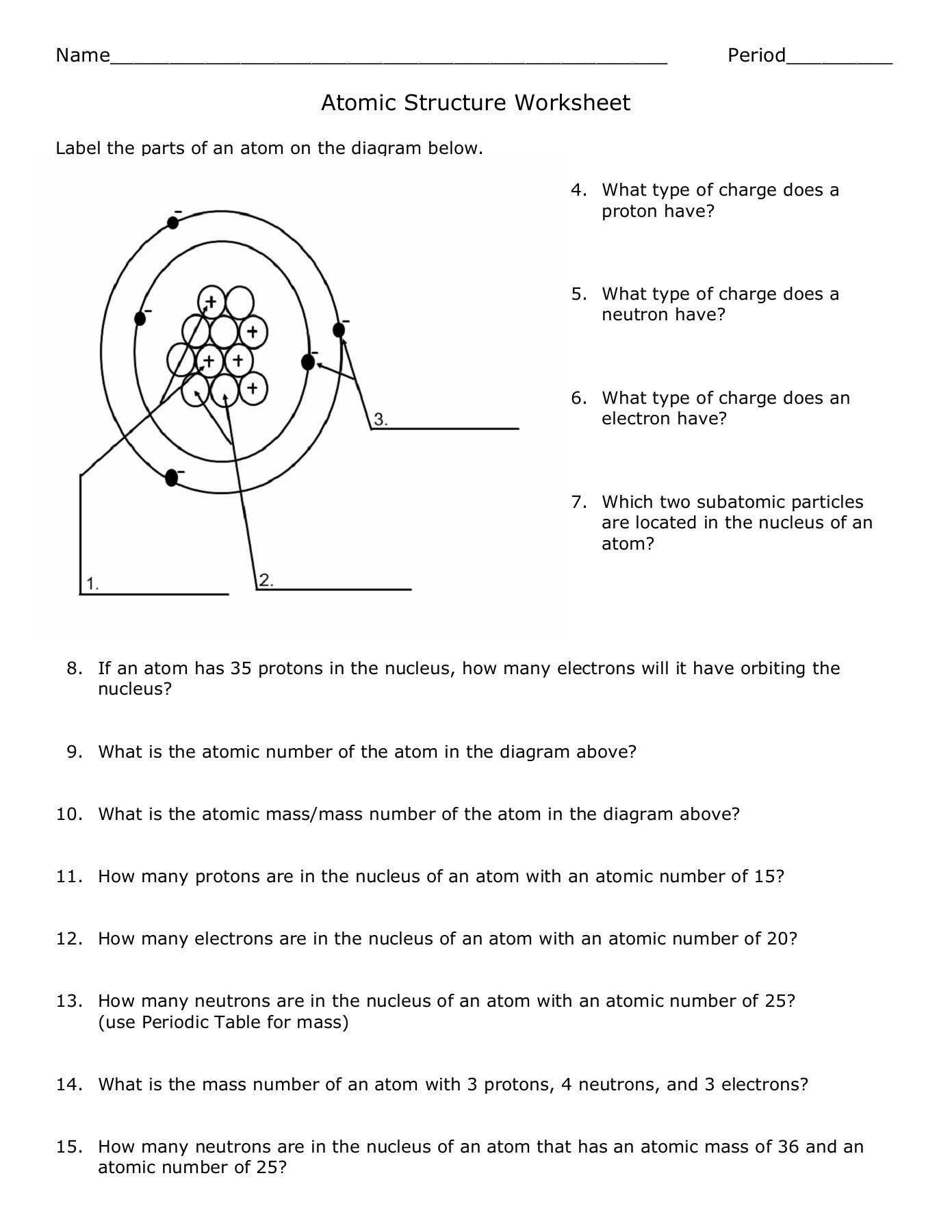
Protons are positively charged particles that reside in the nucleus of an atom. The number of protons in an atom determines the element of an atom, and each element has a unique number of protons in its atoms. Protons have a mass of approximately 1 atomic mass unit (amu).
2. Neutrons
Neutrons are particles that have no charge and are found in the nucleus along with protons. The number of neutrons in an atom can vary, leading to different isotopes of the same element. Neutrons also have a mass of approximately 1 amu.
3. Electrons
Electrons are negatively charged particles that orbit the nucleus of an atom. The number of electrons in an atom is equal to the number of protons, and this number determines the chemical properties of an element. Electrons have a negligible mass compared to protons and neutrons.
4. Nucleus
The nucleus is the central part of an atom that contains the protons and neutrons. It’s incredibly dense, with a diameter of approximately 1⁄100,000 the size of the entire atom.
5. Electron Cloud
The electron cloud refers to the region around the nucleus where electrons are likely to be found. It’s a probability distribution that describes the location of electrons within an atom.
6. Energy Levels
Energy levels, also known as electron shells, are the regions around the nucleus where electrons occupy specific energy states. The energy levels are quantized, meaning that electrons can only occupy specific energy levels.
7. Orbitals
Orbitals are the probability distributions of electrons within an energy level. They describe the shape and orientation of the electron cloud within an atom.
8. Valence Electrons
Valence electrons are the electrons in the outermost energy level of an atom. They participate in chemical bonding and determine the chemical properties of an element.
9. Atomic Mass
The atomic mass is the total mass of an atom, including protons, neutrons, and electrons. It’s a weighted average of the masses of the naturally occurring isotopes of an element.
10. Atomic Number
The atomic number is the number of protons in an atom, which determines the element of an atom. It’s a unique identifier for each element.
🔍 Note: The atomic number and atomic mass are often used interchangeably, but they're not exactly the same thing. The atomic number is a count of protons, while the atomic mass is a weighted average of the masses of the naturally occurring isotopes.
Understanding the 10 essential parts of the atom is crucial for grasping the fundamental principles of chemistry and physics. By knowing the components of an atom, you can better understand chemical bonding, chemical reactions, and the behavior of elements.
What is the difference between an atom and a molecule?
+An atom is the smallest unit of a chemical element, while a molecule is a group of atoms bonded together.
What is the role of electrons in an atom?
+Electrons occupy specific energy levels and participate in chemical bonding, determining the chemical properties of an element.
What is the relationship between protons and neutrons in an atom?
+Protons and neutrons reside in the nucleus, and the number of protons determines the element of an atom. The number of neutrons can vary, leading to different isotopes.
In conclusion, the 10 essential parts of the atom are crucial for understanding the fundamental principles of chemistry and physics. By knowing the components of an atom, you can better understand chemical bonding, chemical reactions, and the behavior of elements.
Related Terms:
- What is an atom Worksheet