Parts of an Atom Worksheet for Science Students
Exploring the Basic Structure of Matter: Understanding the Parts of an Atom
Atoms are the building blocks of matter, and understanding their structure is essential for students of science. This article will delve into the components of an atom, explaining each part in detail, and providing examples and illustrations to reinforce learning.
The Three Main Parts of an Atom
An atom consists of three primary components: protons, neutrons, and electrons. Each of these parts plays a unique role in the atom’s structure and function.
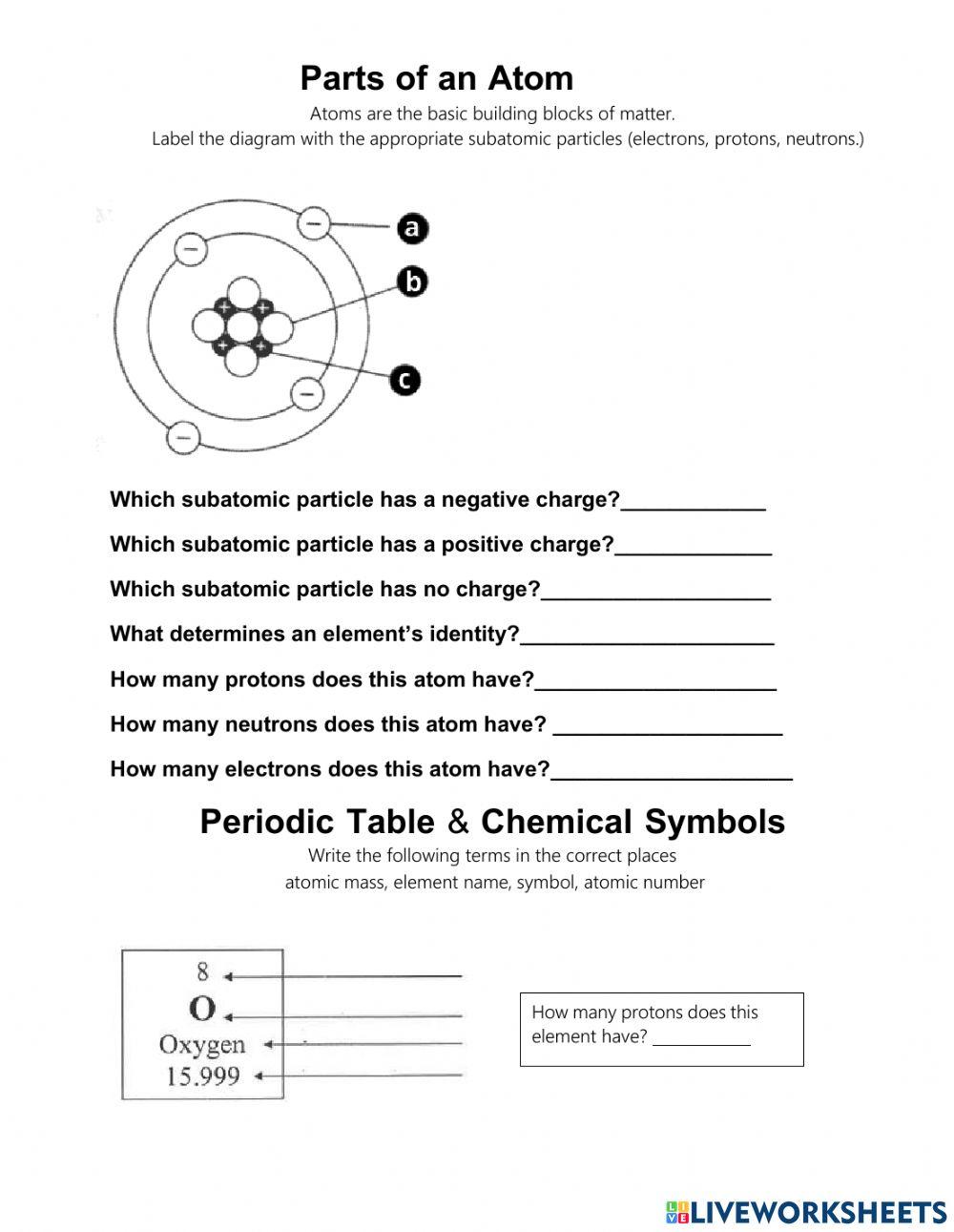
Protons
Protons are positively charged particles that reside in the nucleus (center) of the atom. The number of protons in an atom determines the element of an atom, and each element has a unique number of protons in its atoms. For example, hydrogen has one proton, while oxygen has eight protons.
Neutrons
Neutrons are particles that have no charge and are located in the nucleus along with protons. The number of neutrons in an atom can vary, leading to different isotopes (atoms of the same element with different numbers of neutrons) of an element. Neutrons help stabilize the atom by balancing the positive charge of the protons.
Electrons
Electrons are negatively charged particles that orbit the nucleus of the atom. The number of electrons in an atom is equal to the number of protons, and this number determines the chemical properties of an element. Electrons are arranged in energy levels or electron shells around the nucleus.
Electron Shells and Energy Levels
Electron shells are the regions around the nucleus where electrons are found. The first energy level (or 1s orbital) can hold up to two electrons, while the second energy level (or 2s and 2p orbitals) can hold up to eight electrons. Each energy level has a specific capacity, and electrons fill the lowest available energy levels first.

| Energy Level | Electron Capacity |
|---|---|
| 1 (1s) | 2 |
| 2 (2s and 2p) | 8 |
| 3 (3s, 3p, and 3d) | 18 |
Atomic Number and Mass Number
The atomic number of an atom is the number of protons in its nucleus, which determines the element of an atom. The mass number is the total number of protons and neutrons in the nucleus.
📝 Note: The mass number is also known as the atomic mass.
Conclusion
In conclusion, understanding the parts of an atom is crucial for science students. By grasping the concepts of protons, neutrons, electrons, electron shells, and energy levels, students can better comprehend the structure and properties of matter.
What is the main difference between protons and neutrons?
+Protons have a positive charge, while neutrons have no charge.
What determines the chemical properties of an element?
+The number of electrons in an atom determines the chemical properties of an element.
What is the relationship between the atomic number and the mass number?
+The atomic number is the number of protons, while the mass number is the total number of protons and neutrons.