Mastering Nuclear Equations Worksheet Solutions
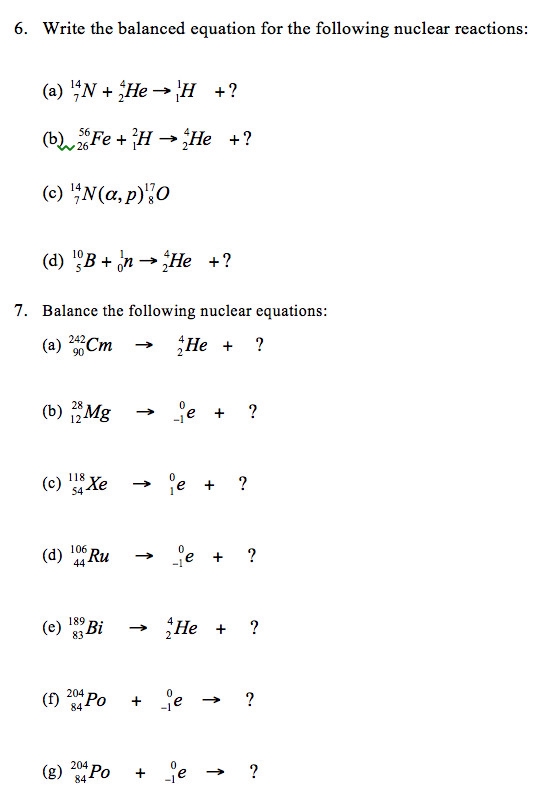
Introduction to Nuclear Equations
Nuclear equations are a crucial part of nuclear physics, and they play a vital role in understanding various nuclear reactions. These equations are used to describe the changes that occur in the nucleus of an atom during a nuclear reaction. In this article, we will provide solutions to some common nuclear equations worksheet problems.
Types of Nuclear Equations
There are several types of nuclear equations, including:
- Radioactive Decay Equations: These equations describe the decay of unstable nuclei into more stable ones.
- Nuclear Fission Equations: These equations describe the splitting of heavy nuclei into lighter ones.
- Nuclear Fusion Equations: These equations describe the combination of light nuclei to form heavier ones.
Solutions to Nuclear Equations Worksheet Problems
Problem 1: Radioactive Decay
Complete the following nuclear equation:
²³⁸U → ²³⁴Th +?
Solution
To balance the equation, we need to ensure that the mass numbers and atomic numbers are equal on both sides. Since ²³⁸U has a mass number of 238 and an atomic number of 92, the product should have a mass number of 234 and an atomic number of 90.
²³⁸U → ²³⁴Th + ⁴He
Problem 2: Nuclear Fission
Write the nuclear equation for the fission of ²³⁵U into ¹⁴¹Ba and ⁹²Kr.
Solution
To write the equation, we need to ensure that the mass numbers and atomic numbers are equal on both sides. Since ²³⁵U has a mass number of 235 and an atomic number of 92, the products should have mass numbers of 141 and 92, and atomic numbers of 56 and 36, respectively.
²³⁵U → ¹⁴¹Ba + ⁹²Kr + 3n
Problem 3: Nuclear Fusion
Write the nuclear equation for the fusion of ²H and ³H into ⁴He.
Solution
To write the equation, we need to ensure that the mass numbers and atomic numbers are equal on both sides. Since ²H has a mass number of 2 and an atomic number of 1, and ³H has a mass number of 3 and an atomic number of 1, the product should have a mass number of 4 and an atomic number of 2.
²H + ³H → ⁴He + n
Notes
📝 Note: In nuclear equations, the mass numbers and atomic numbers must be equal on both sides. This is a fundamental principle of nuclear physics.
Conclusion
Mastering nuclear equations is a crucial part of understanding nuclear physics. By solving various types of nuclear equations, you can gain a deeper understanding of the changes that occur in the nucleus of an atom during a nuclear reaction. Remember to always balance the mass numbers and atomic numbers on both sides of the equation.
What is the purpose of nuclear equations?
+Nuclear equations are used to describe the changes that occur in the nucleus of an atom during a nuclear reaction.
What are the different types of nuclear equations?
+There are several types of nuclear equations, including radioactive decay equations, nuclear fission equations, and nuclear fusion equations.
How do you balance a nuclear equation?
+To balance a nuclear equation, you need to ensure that the mass numbers and atomic numbers are equal on both sides.