5 Nuclear Chemistry Review Essentials
Understanding the Fundamentals of Nuclear Chemistry
Nuclear chemistry is a branch of chemistry that deals with the study of changes in the nucleus of an atom. This field of chemistry is crucial in understanding various phenomena, from nuclear power generation to medical applications. As we explore the essentials of nuclear chemistry, it is vital to grasp the key concepts, principles, and applications that make up this fascinating field.
Radioactivity and Nuclear Stability
Radioactivity is a process by which unstable atomic nuclei lose energy through radiation. This phenomenon was first discovered by Henri Becquerel in 1896. Radioactivity is a natural process that occurs in unstable isotopes, which can be found in various elements. Understanding radioactivity is essential in nuclear chemistry, as it helps us comprehend the stability of atomic nuclei.
Key Factors Affecting Nuclear Stability:
- Neutron-to-proton ratio
- Number of protons and neutrons
- Binding energy per nucleon
⚠️ Note: Nuclear stability is crucial in determining the radioactivity of an element.
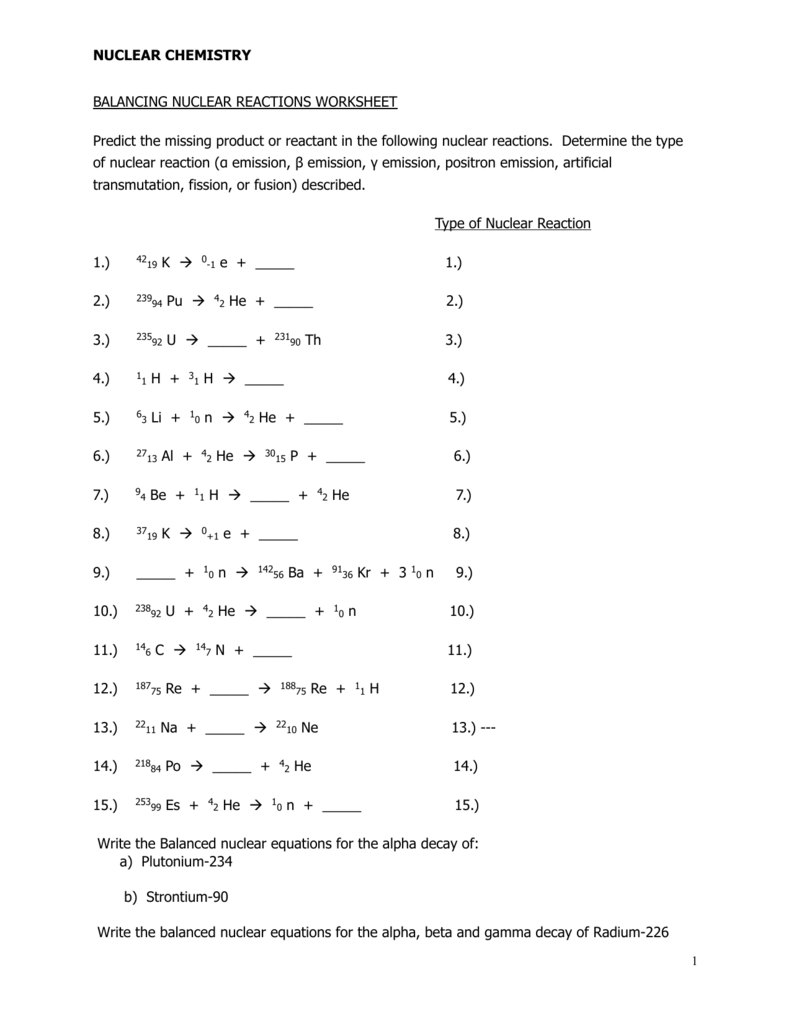
Nuclear Reactions and Equations
Nuclear reactions involve changes in the nucleus of an atom, resulting in the transformation of one element into another. These reactions can be represented by nuclear equations, which follow specific rules and conventions. Understanding nuclear equations is vital in predicting and analyzing nuclear reactions.
Types of Nuclear Reactions:
- Radioactive decay
- Nuclear fission
- Nuclear fusion
- Neutron-induced reactions
| Nuclear Reaction | Example |
|---|---|
| Radioactive decay | 238U → 234Th + α |
| Nuclear fission | 235U + n → 141Ba + 92Kr + 3n |
| Nuclear fusion | 2H + 3H → 4He + n |
| Neutron-induced reaction | 235U + n → 236U |
Nuclear Energy and Applications
Nuclear energy is a significant source of electricity worldwide, accounting for approximately 10% of global electricity generation. Nuclear power plants harness the energy released from nuclear fission reactions to produce steam, which drives turbines to generate electricity.
Medical Applications of Nuclear Chemistry:
- Cancer treatment (radiotherapy)
- Imaging techniques (PET scans)
- Diagnostic tools (radioimmunoassays)
💡 Note: Nuclear chemistry has numerous medical applications, improving diagnosis and treatment of various diseases.
Nuclear Safety and Waste Management
Nuclear safety is a critical concern in the nuclear industry, as it involves the handling of radioactive materials and the potential risks associated with nuclear reactions. Proper waste management is also essential in minimizing the environmental impact of nuclear activities.
Key Principles of Nuclear Safety:
- Containment
- Shielding
- Cooling
- Emergency preparedness
🚨 Note: Nuclear safety is a top priority in the nuclear industry, and adherence to strict guidelines is crucial in preventing accidents.
In conclusion, nuclear chemistry is a complex and fascinating field that requires a deep understanding of its fundamental principles and applications. By grasping the essentials of nuclear chemistry, we can better appreciate the importance of this field in various aspects of our lives.
What is nuclear chemistry?
+Nuclear chemistry is a branch of chemistry that deals with the study of changes in the nucleus of an atom.
What are the key factors affecting nuclear stability?
+The key factors affecting nuclear stability include the neutron-to-proton ratio, the number of protons and neutrons, and the binding energy per nucleon.
What are some medical applications of nuclear chemistry?
+Nuclear chemistry has numerous medical applications, including cancer treatment (radiotherapy), imaging techniques (PET scans), and diagnostic tools (radioimmunoassays).