Mole Worksheet 1 Answer Key Simplified
Understanding the Concept of Moles
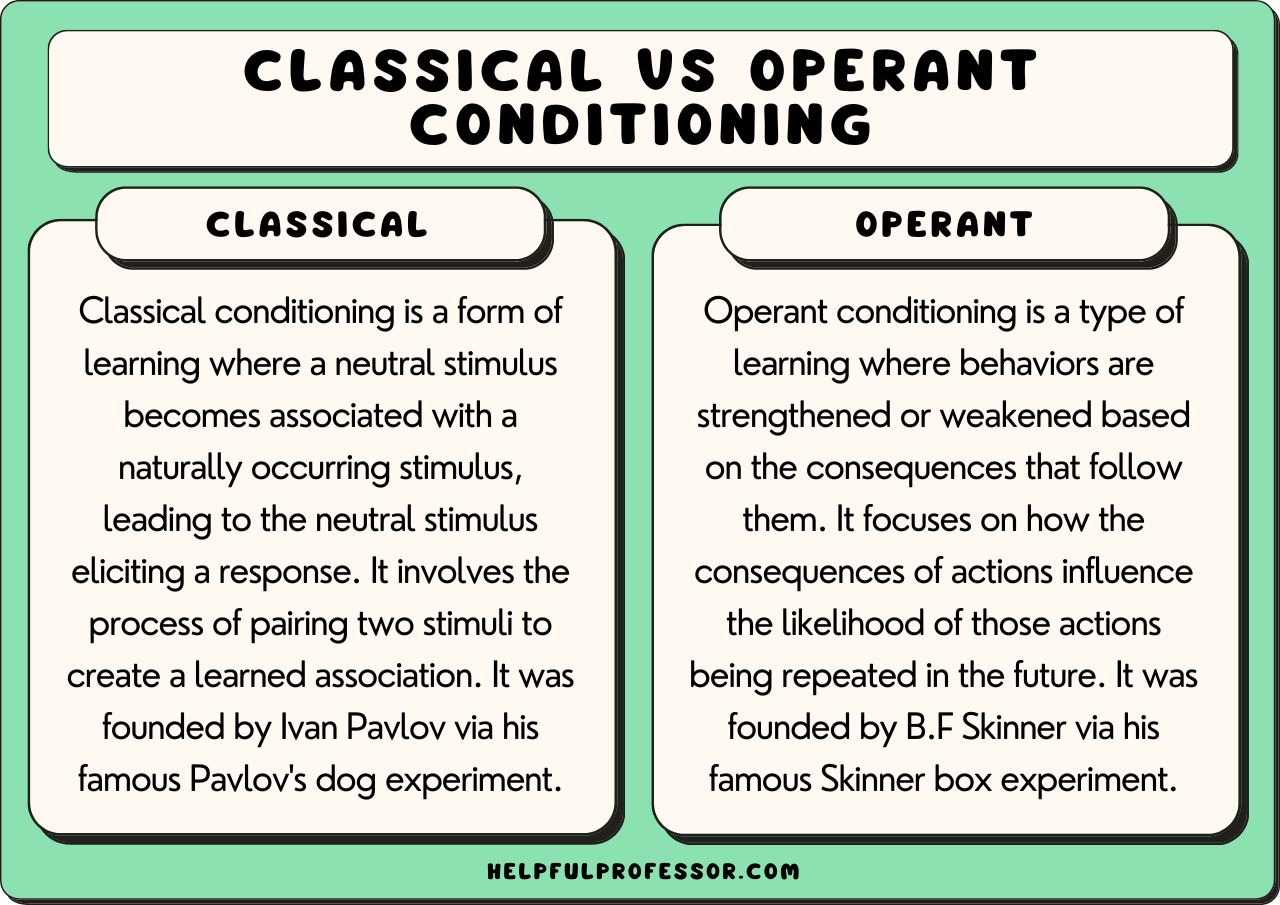
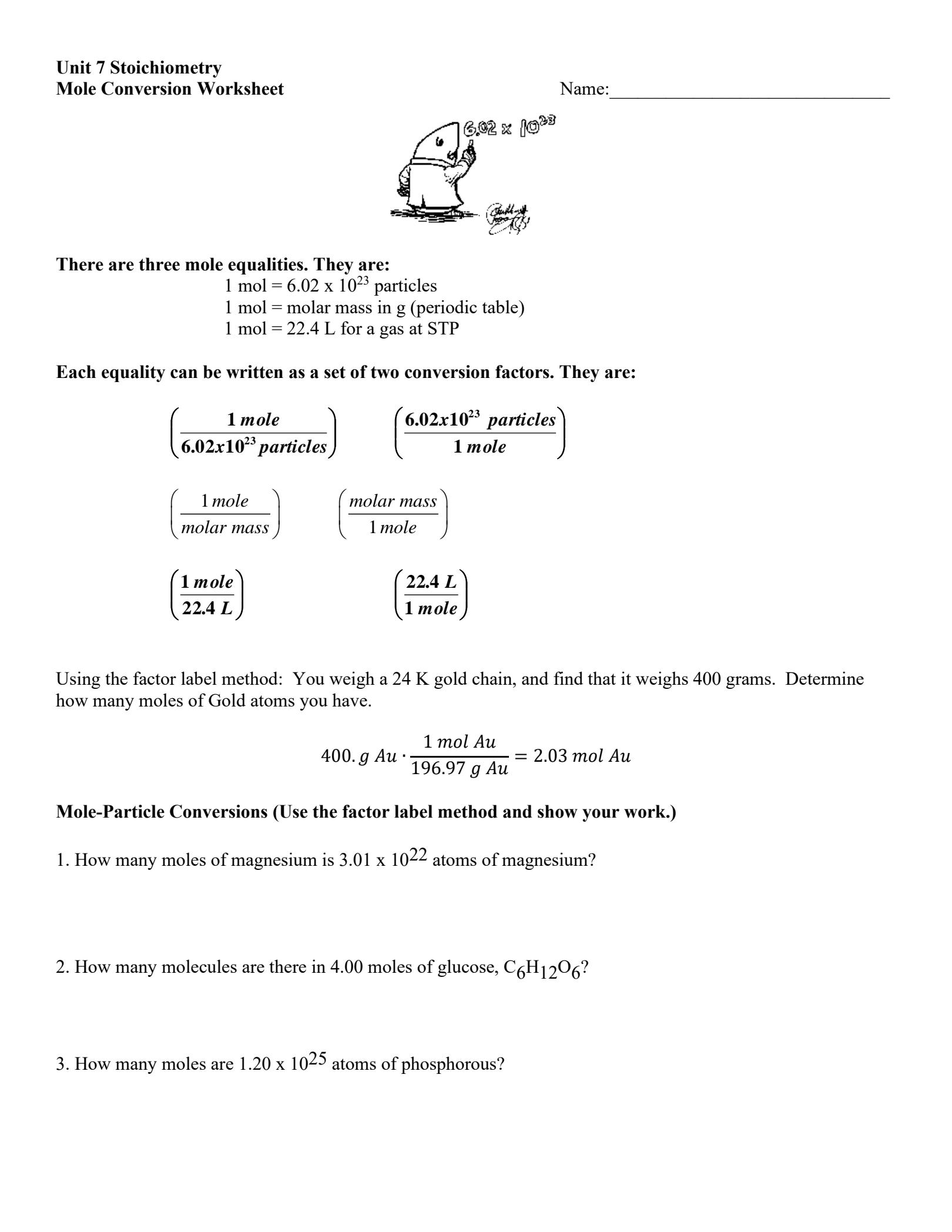
The mole is a fundamental unit in chemistry that represents 6.022 x 10^23 particles, such as atoms or molecules. This concept is crucial in quantitative chemistry, as it allows us to easily calculate the amounts of substances involved in chemical reactions.
What is a Mole?
A mole is defined as the amount of substance that contains as many particles (atoms, molecules, or formula units) as there are atoms in 0.012 kilograms of carbon-12. This number is known as the Avogadro’s constant, which is approximately 6.022 x 10^23 particles.
Mole Calculations
To solve mole-related problems, you need to understand the relationship between the number of moles, the molar mass, and the number of particles. Here are some key formulas to keep in mind:
- Number of moles (n) = mass of substance (m) / molar mass (M)
- Number of particles (N) = number of moles (n) x Avogadro’s constant (NA)
Example Problem 1
Calculate the number of moles of oxygen molecules in 32 grams of oxygen gas.
📝 Note: The molar mass of oxygen gas (O2) is 32 g/mol.
Solution: Number of moles (n) = mass of substance (m) / molar mass (M) = 32 g / 32 g/mol = 1 mol
Example Problem 2
How many particles are in 2 moles of carbon dioxide molecules?
Solution: Number of particles (N) = number of moles (n) x Avogadro’s constant (NA) = 2 mol x 6.022 x 10^23 particles/mol = 1.2044 x 10^24 particles
Converting Between Moles and Mass
To convert between moles and mass, you can use the following formulas:
- Mass (m) = number of moles (n) x molar mass (M)
- Number of moles (n) = mass (m) / molar mass (M)
Example Problem 3
Calculate the mass of 0.5 moles of sodium chloride.
💡 Note: The molar mass of sodium chloride (NaCl) is 58.44 g/mol.
Solution: Mass (m) = number of moles (n) x molar mass (M) = 0.5 mol x 58.44 g/mol = 29.22 g
Real-World Applications of Moles
The concept of moles has numerous real-world applications, including:
- Medicinal dosages: Moles are used to calculate the correct dosage of medications.
- Food production: Moles are used to calculate the amount of ingredients needed for food production.
- Environmental monitoring: Moles are used to calculate the amount of pollutants in the environment.
In Conclusion
Mastering the concept of moles is essential for understanding quantitative chemistry. By understanding the relationship between moles, molar mass, and number of particles, you can solve a wide range of problems in chemistry.
What is the definition of a mole?
+A mole is defined as the amount of substance that contains as many particles (atoms, molecules, or formula units) as there are atoms in 0.012 kilograms of carbon-12.
How do I calculate the number of moles of a substance?
+Number of moles (n) = mass of substance (m) / molar mass (M)
What is the Avogadro’s constant?
+The Avogadro’s constant is approximately 6.022 x 10^23 particles.