5 Ways to Balance Chemical Equations Easily
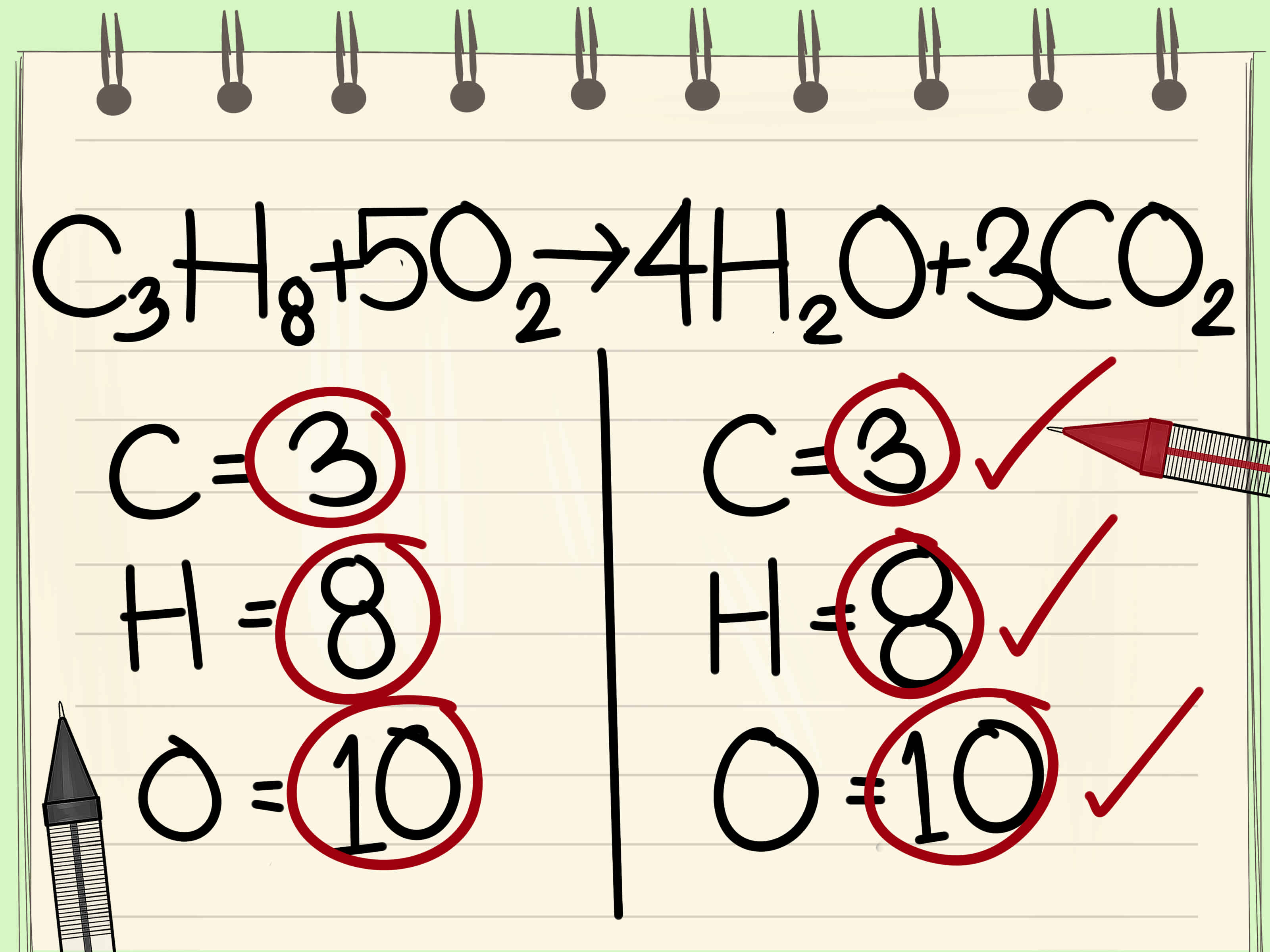
Understanding the Basics of Chemical Equations
Chemical equations are a fundamental part of chemistry, representing the reactants, products, and direction of a chemical reaction. These equations are balanced to ensure the law of conservation of mass is upheld, meaning the number of atoms of each element is the same on both the reactant and product sides. Balancing chemical equations can be a daunting task, especially for beginners. However, with practice and the right strategies, it can become easier. Here are five ways to balance chemical equations easily.
Method 1: The Trial and Error Method
The trial and error method involves adding coefficients (numbers in front of the formulas of reactants or products) to balance the equation. This method can be time-consuming and requires patience, but it is straightforward. Here’s how to do it:
- Step 1: Write down the unbalanced equation.
- Step 2: Identify the elements that are not balanced.
- Step 3: Add coefficients in front of the formulas of reactants or products to balance the elements, starting with elements that appear only once on each side.
- Step 4: Check the equation to ensure all elements are balanced.
- Step 5: Repeat the process until the equation is balanced.
💡 Note: This method is suitable for simple equations but can be cumbersome for complex reactions.
Method 2: The Half-Reaction Method
The half-reaction method is more systematic and is particularly useful for redox reactions. This method involves breaking down the reaction into two half-reactions: oxidation and reduction. Here’s how to apply it:
- Step 1: Break down the reaction into two half-reactions.
- Step 2: Balance each half-reaction separately, starting with the elements and then the charges.
- Step 3: Combine the balanced half-reactions.
- Step 4: Add coefficients as necessary to ensure the equation is fully balanced.
⚠️ Note: Ensure that the number of electrons lost in the oxidation half-reaction equals the number of electrons gained in the reduction half-reaction.
Method 3: The Algebraic Method
The algebraic method involves assigning variables to the coefficients of the reactants and products and solving the resulting system of equations. Here’s how to apply it:
- Step 1: Assign variables to the coefficients.
- Step 2: Write down the system of equations based on the conservation of mass.
- Step 3: Solve the system of equations.
- Step 4: Substitute the values back into the original equation to get the balanced equation.
📝 Note: This method requires a basic understanding of algebra and can be complex for large systems of equations.
Method 4: The Inspection Method
The inspection method involves looking at the equation and making educated guesses about the coefficients. This method requires experience and a deep understanding of chemistry. Here’s how to apply it:
- Step 1: Look at the equation and identify patterns or common combinations.
- Step 2: Make educated guesses about the coefficients based on experience and knowledge.
- Step 3: Check the equation to ensure it is balanced.
👓 Note: This method is best suited for experienced chemists and can be unreliable for complex reactions.
Method 5: Using Online Tools
With the advent of technology, there are now many online tools and software that can balance chemical equations automatically. Here’s how to use them:
- Step 1: Choose a reliable online tool or software.
- Step 2: Enter the unbalanced equation into the tool.
- Step 3: The tool will generate the balanced equation.
🖥️ Note: While these tools can save time, they should not replace learning the fundamental methods of balancing chemical equations.
Chemical equations are a fundamental part of chemistry, and balancing them is a crucial skill for any chemist. By mastering these five methods, you can easily balance chemical equations and take your chemistry skills to the next level.
Balancing chemical equations is a skill that requires practice and patience. By combining these methods and practicing regularly, you can become proficient in balancing even the most complex chemical equations.
What is the most common method of balancing chemical equations?
+
The trial and error method is the most common method of balancing chemical equations. However, the half-reaction method is also widely used, especially for redox reactions.
Can online tools replace traditional methods of balancing chemical equations?
+
No, online tools should not replace traditional methods of balancing chemical equations. While they can save time, it’s essential to understand the fundamental methods to truly master the skill.
What is the importance of balancing chemical equations?
+
Balancing chemical equations is crucial to ensure the law of conservation of mass is upheld. It’s also essential for predicting the amounts of reactants and products in a chemical reaction.