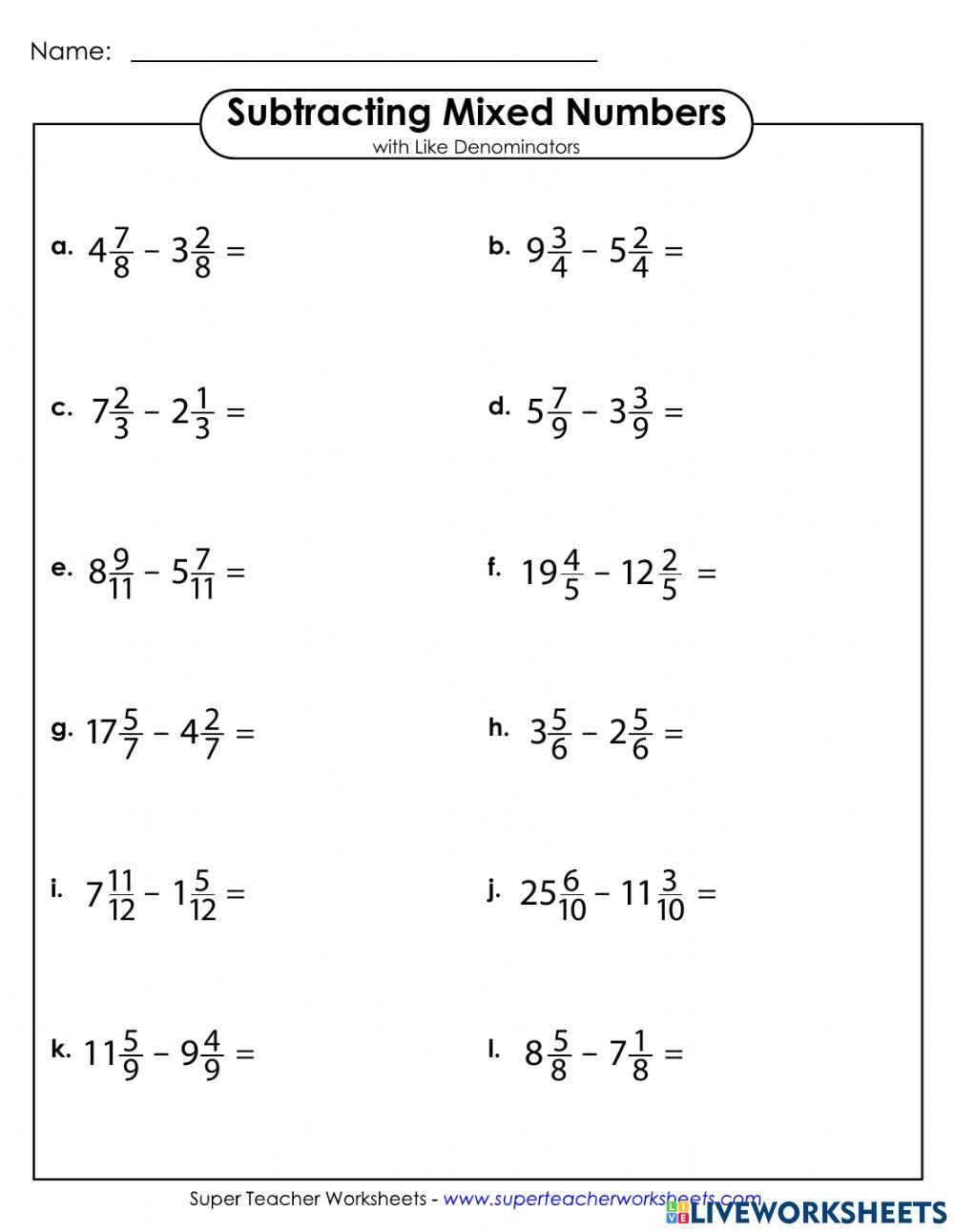
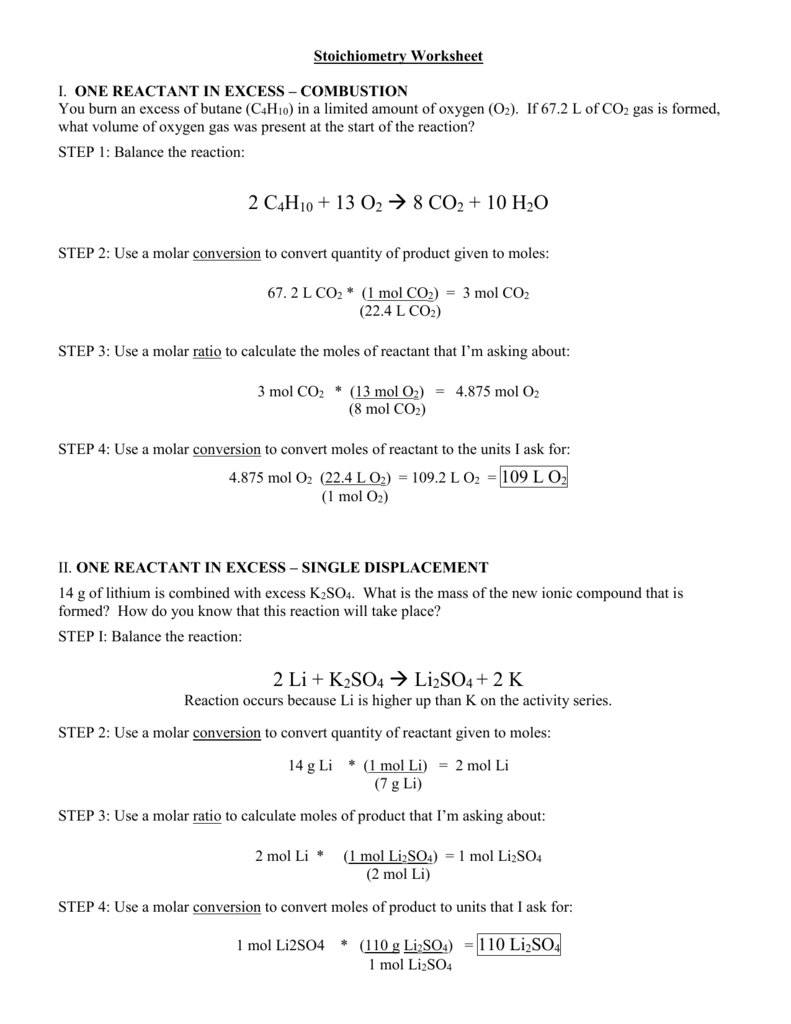
5 Ways to Master Mole Ratios
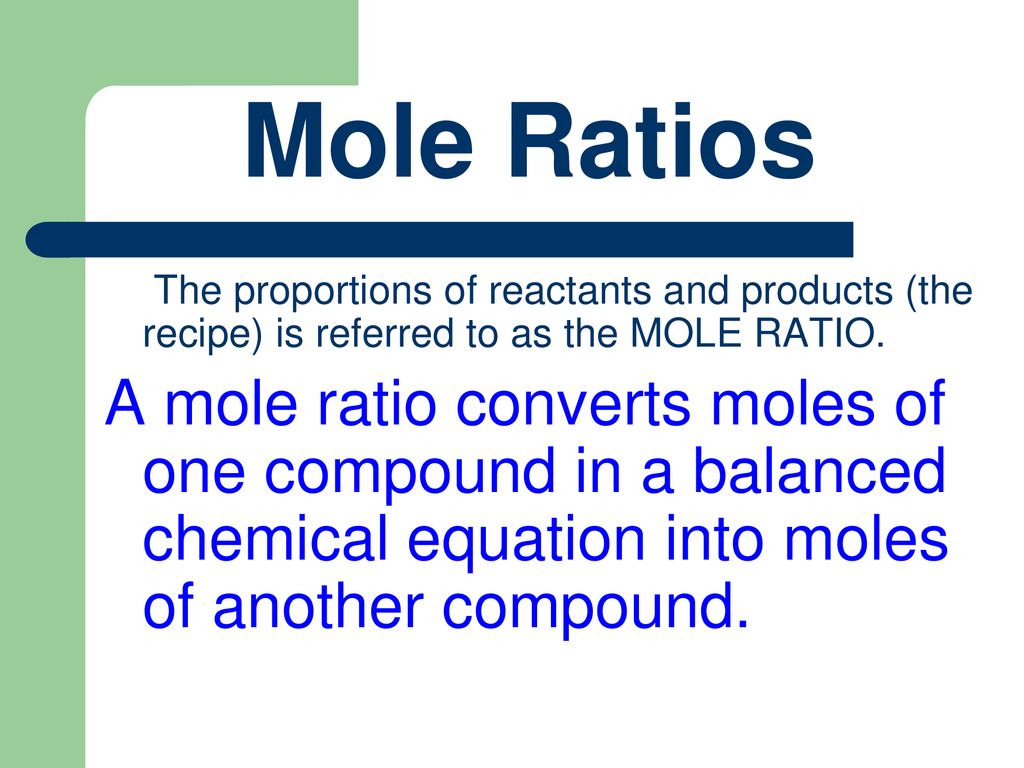
Mastering mole ratios is a fundamental skill in chemistry, and it’s essential to understand how to work with them to solve problems. In this article, we’ll explore five ways to master mole ratios and provide you with the skills and confidence to tackle even the most challenging problems.
Understanding Mole Ratios
Before we dive into the five ways to master mole ratios, let’s quickly review what a mole ratio is. A mole ratio is a way of expressing the relationship between the number of moles of one substance and the number of moles of another substance in a chemical reaction. It’s a ratio of the number of moles of each reactant or product, and it’s usually expressed as a simple fraction.
For example, consider the following equation:
2H2 + O2 → 2H2O
The mole ratio of hydrogen gas to oxygen gas is 2:1, which means that for every 2 moles of hydrogen gas, there is 1 mole of oxygen gas.
1. Practice, Practice, Practice
The first way to master mole ratios is to practice, practice, practice! The more you practice working with mole ratios, the more comfortable you’ll become with them. Start with simple problems, such as converting between moles and grams, and then move on to more complex problems, such as balancing equations and calculating mole ratios.
Here are some tips to help you practice:
- Start with simple problems and gradually increase the difficulty level.
- Use online resources, such as worksheets and practice problems, to help you practice.
- Practice regularly, even if it’s just for a few minutes each day.
2. Use Real-World Examples
The second way to master mole ratios is to use real-world examples. Using real-world examples can help make mole ratios more meaningful and interesting. For example, consider the following problem:
A bakery uses 2.5 kg of flour to make 100 loaves of bread. If the molecular weight of flour is 50 g/mol, how many moles of flour are used to make 100 loaves of bread?
This problem is more interesting and meaningful than a simple problem that just asks you to calculate a mole ratio. By using real-world examples, you can make mole ratios more relevant and engaging.
3. Focus on the Basics
The third way to master mole ratios is to focus on the basics. Make sure you understand the basics of mole ratios, such as how to calculate them and how to use them to solve problems. Don’t try to learn too much too quickly – focus on building a strong foundation in mole ratios.
Here are some key concepts to focus on:
- Mole ratio definition: A mole ratio is a ratio of the number of moles of one substance to the number of moles of another substance.
- How to calculate mole ratios: To calculate a mole ratio, divide the number of moles of one substance by the number of moles of another substance.
- How to use mole ratios: Mole ratios can be used to solve problems, such as balancing equations and calculating the amount of a substance needed.
4. Use Visual Aids
The fourth way to master mole ratios is to use visual aids. Visual aids, such as diagrams and charts, can help you understand and work with mole ratios more effectively. For example, consider the following diagram:
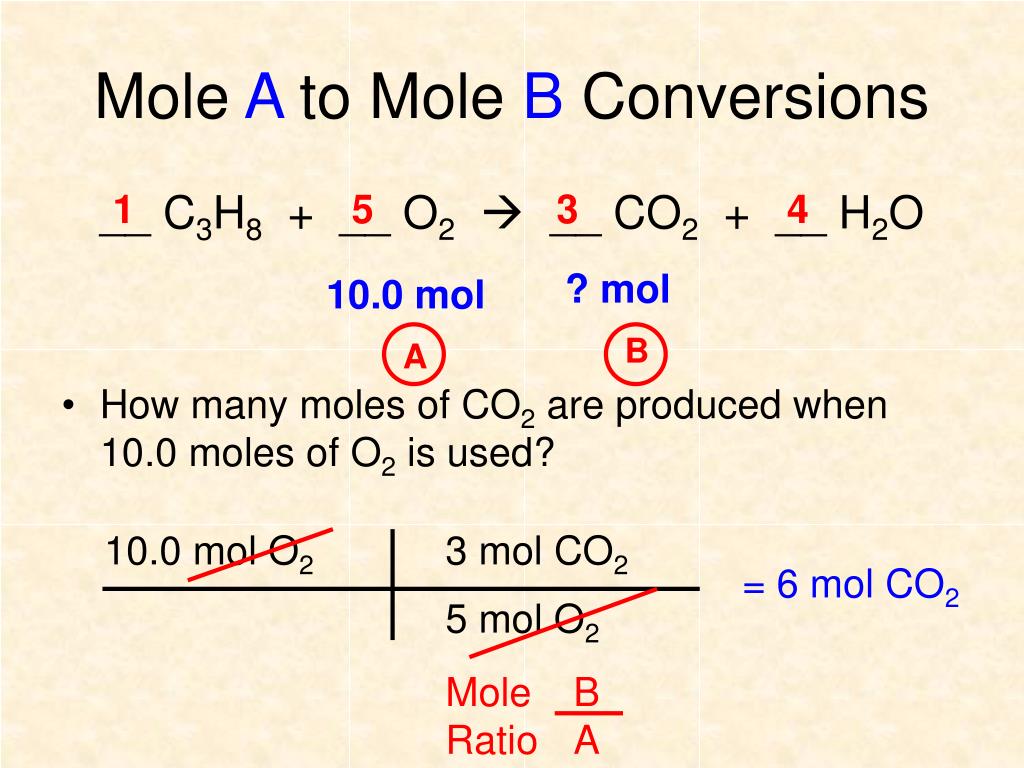
| Substance | Moles |
|---|---|
| H2 | 2 |
| O2 | 1 |
| H2O | 2 |
This diagram shows the mole ratio of hydrogen gas to oxygen gas to water. By using visual aids like this, you can more easily see the relationships between different substances and calculate mole ratios.
5. Check Your Work
The fifth way to master mole ratios is to check your work. When working with mole ratios, it’s easy to make mistakes. To avoid mistakes, check your work carefully. Here are some tips to help you check your work:
- Use multiple methods: Use multiple methods to check your work, such as plugging in numbers to see if the equation balances.
- Use online resources: Use online resources, such as calculators and worksheets, to check your work.
- Check your units: Make sure your units are correct and consistent.
🤔 Note: When checking your work, make sure to double-check your calculations and units. A small mistake can lead to big errors!
To summarize, mastering mole ratios requires practice, real-world examples, a focus on the basics, visual aids, and careful checking of your work. By following these five tips, you’ll be well on your way to becoming a master of mole ratios!
In conclusion, mole ratios are a fundamental concept in chemistry, and mastering them is essential for success in the field. By practicing regularly, using real-world examples, focusing on the basics, using visual aids, and checking your work carefully, you can become proficient in working with mole ratios. Remember to stay calm and patient, and don’t be afraid to ask for help when you need it.
What is a mole ratio?
+A mole ratio is a ratio of the number of moles of one substance to the number of moles of another substance in a chemical reaction.
How do I calculate a mole ratio?
+To calculate a mole ratio, divide the number of moles of one substance by the number of moles of another substance.
Why is it important to check my work when working with mole ratios?
+Checking your work is important to avoid mistakes and ensure that your calculations are accurate.