5 Easy Ways to Solve Molarity Problems
Understanding Molarity: A Crucial Concept in Chemistry
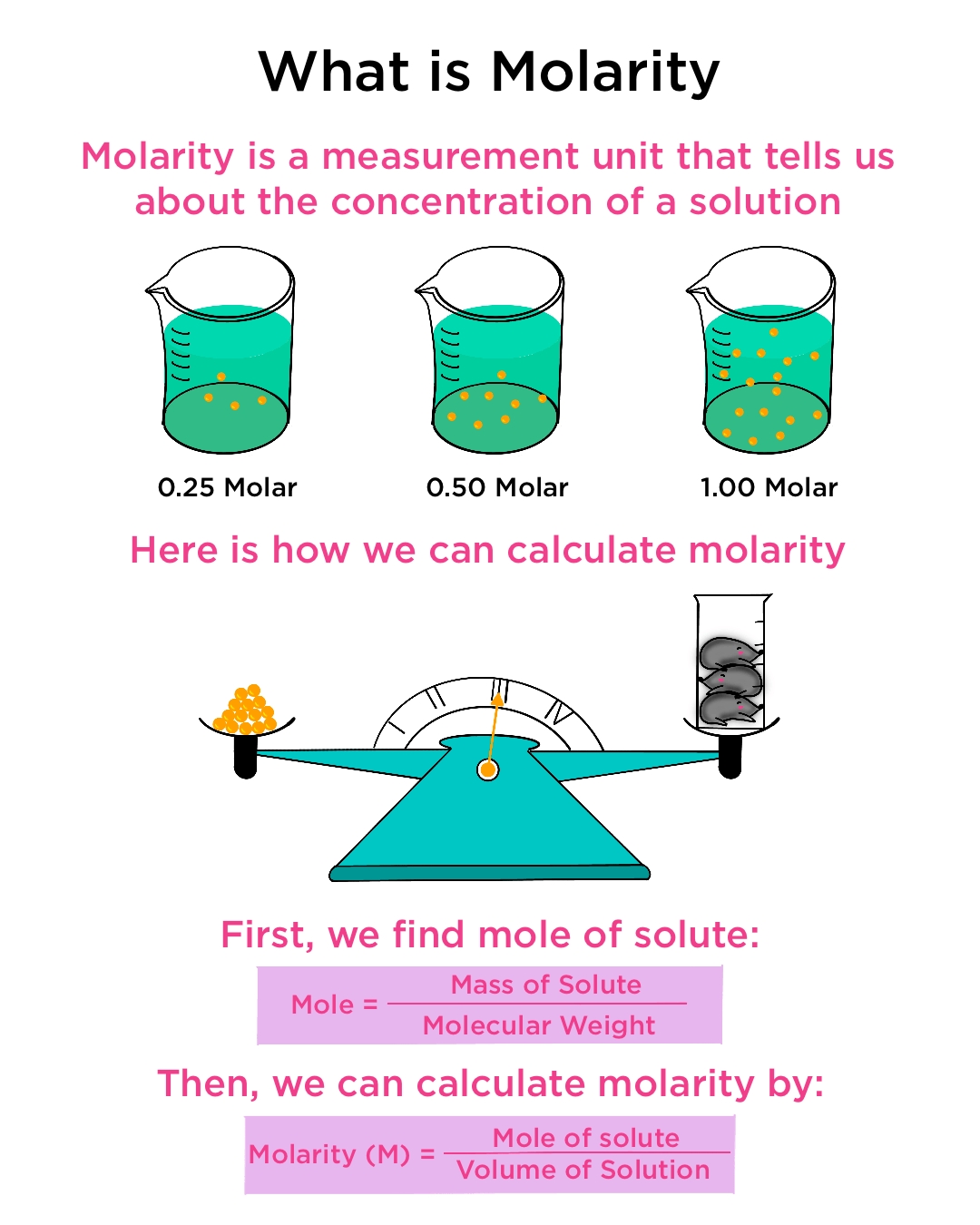
Molarity is a fundamental concept in chemistry that measures the concentration of a solution. It is defined as the number of moles of a substance dissolved in one liter of a solution. Molarity problems can be challenging, but with the right approach, they can be solved easily. In this article, we will explore five easy ways to solve molarity problems.
Method 1: Using the Molarity Formula
The molarity formula is a straightforward way to solve molarity problems. The formula is:
Molarity (M) = Number of moles of solute / Volume of solution in liters (L)
M = moles / L
To use this formula, you need to know the number of moles of the solute and the volume of the solution in liters.
Example: What is the molarity of a solution that contains 2 moles of sodium chloride (NaCl) in 4 liters of water?
M = 2 moles / 4 L = 0.5 M
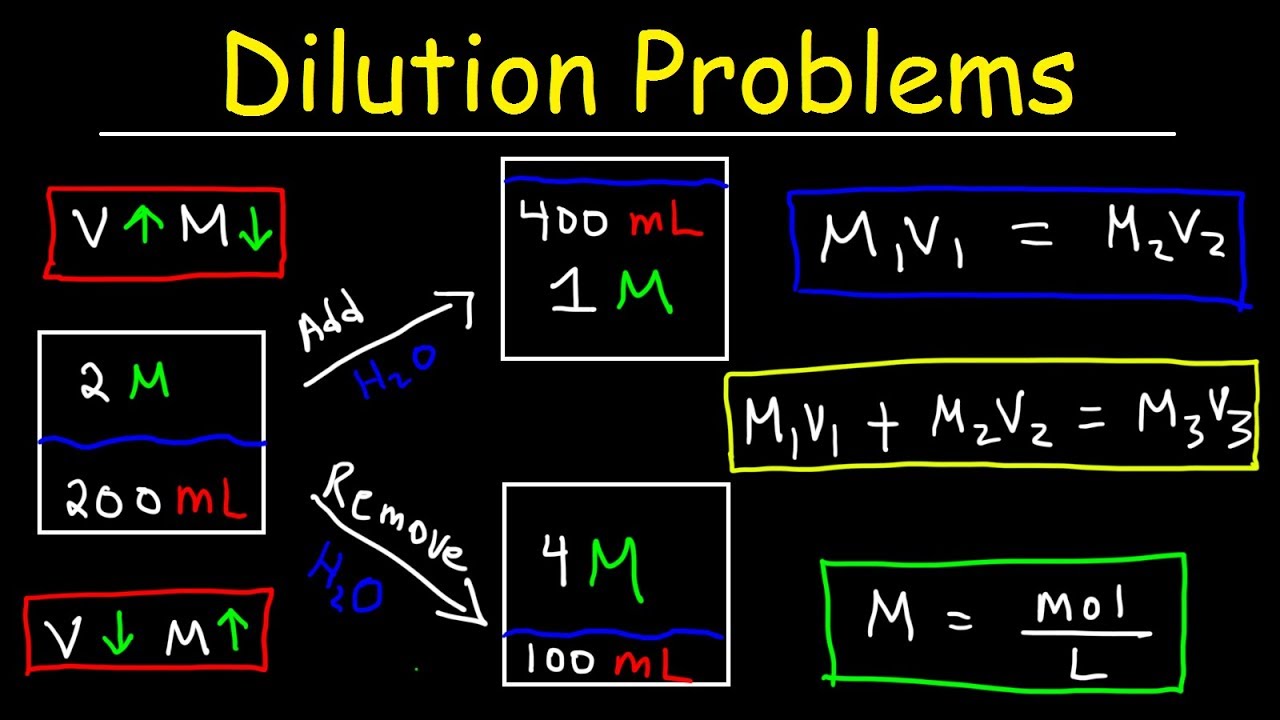
Method 2: Using the Dilution Formula
The dilution formula is used to solve problems involving the dilution of a solution. The formula is:
M1V1 = M2V2
Where: M1 = initial molarity V1 = initial volume M2 = final molarity V2 = final volume
To use this formula, you need to know the initial molarity and volume, as well as the final volume.
Example: If 2 liters of a 1 M solution of hydrochloric acid (HCl) is diluted to 4 liters, what is the final molarity?
M1V1 = M2V2 1 M x 2 L = M2 x 4 L M2 = 0.5 M
Method 3: Using the Percentage Composition
The percentage composition of a solution can be used to solve molarity problems. The formula is:
Molarity (M) = (Percentage composition / 100) x (Density of solution / Molecular weight of solute)
To use this formula, you need to know the percentage composition, density of the solution, and molecular weight of the solute.
Example: What is the molarity of a solution that contains 20% sodium hydroxide (NaOH) by weight, with a density of 1.2 g/mL and a molecular weight of 40 g/mol?
M = (20 / 100) x (1.2 g/mL / 40 g/mol) = 0.06 M
Method 4: Using the Normality Formula
Normality is another measure of concentration that is related to molarity. The formula is:
Normality (N) = Number of equivalents of solute / Volume of solution in liters (L)
To use this formula, you need to know the number of equivalents of the solute and the volume of the solution in liters.
Example: What is the normality of a solution that contains 2 equivalents of sulfuric acid (H2SO4) in 4 liters of water?
N = 2 equivalents / 4 L = 0.5 N
Since normality is related to molarity, you can convert normality to molarity using the following formula:
Molarity (M) = Normality (N) / Equivalent weight of solute
Example: What is the molarity of the solution in the previous example, given that the equivalent weight of sulfuric acid is 49 g/mol?
M = 0.5 N / 49 g/mol = 0.01 M
Method 5: Using a Molarity Table
A molarity table is a useful tool for solving molarity problems. It is a table that lists the molarity of a solution as a function of the volume of the solute and the volume of the solution.
Example: What is the molarity of a solution that contains 2 mL of 1 M sodium chloride (NaCl) in 4 liters of water?
Using a molarity table, you can look up the molarity of the solution based on the volume of the solute and the volume of the solution.
| Volume of solute (mL) | Volume of solution (L) | Molarity (M) |
|---|---|---|
| 2 | 4 | 0.05 |
As you can see, the molarity of the solution is 0.05 M.
📝 Note: When using a molarity table, make sure to check the units of the volumes and the molarity to ensure that they match.
In conclusion, solving molarity problems can be easy and straightforward if you know the right approach. By using the molarity formula, dilution formula, percentage composition, normality formula, or a molarity table, you can solve a wide range of molarity problems. Remember to always check your units and ensure that you have the correct information before solving a problem.
What is molarity?
+Molarity is a measure of the concentration of a solution, defined as the number of moles of a substance dissolved in one liter of a solution.
What is the difference between molarity and normality?
+Molarity and normality are both measures of concentration, but they differ in their units and applications. Molarity is measured in moles per liter, while normality is measured in equivalents per liter.
How do I convert normality to molarity?
+To convert normality to molarity, divide the normality by the equivalent weight of the solute.