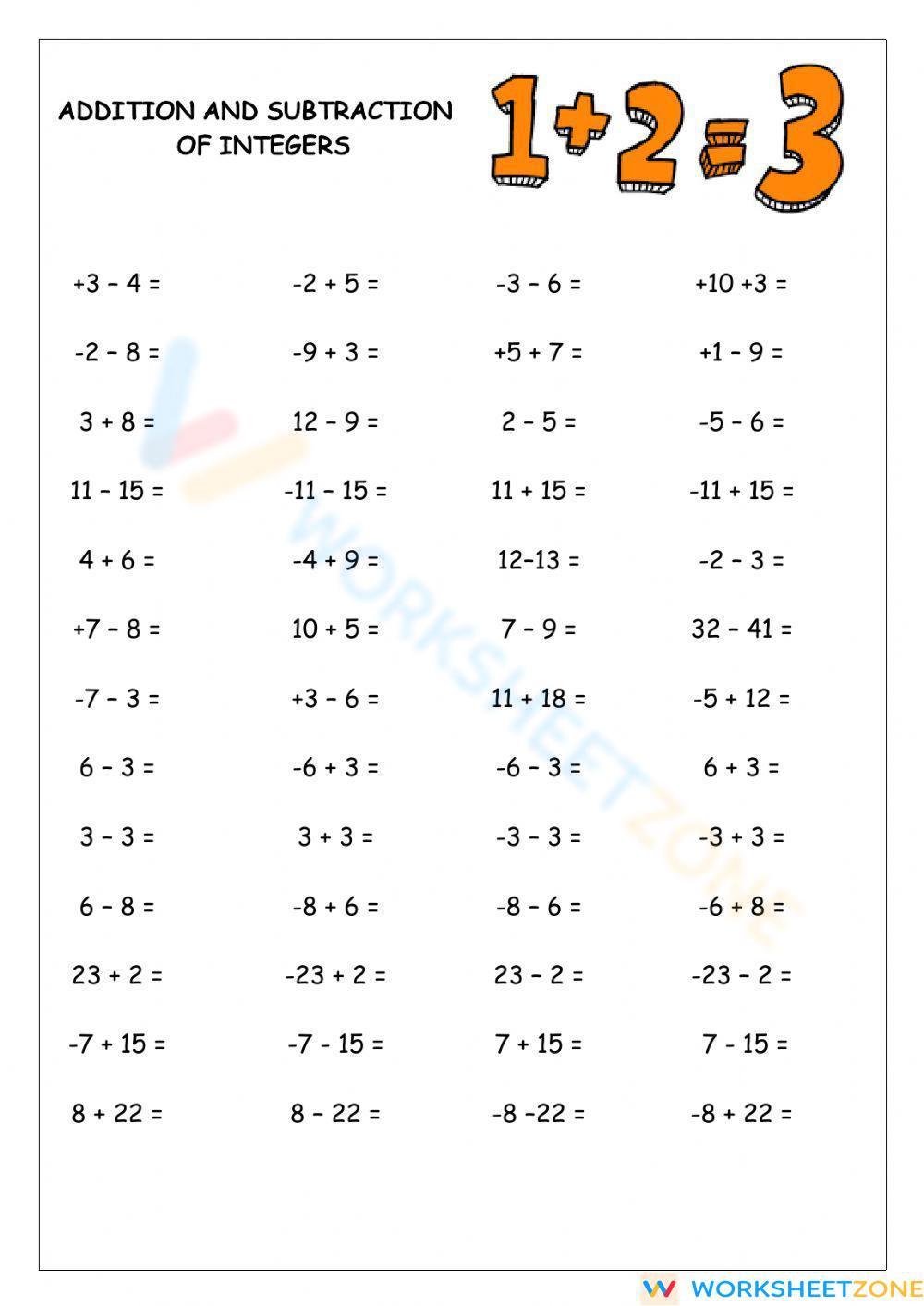
Naming Compounds Made Easy with This Worksheet Guide
Naming Compounds: A Comprehensive Guide
When it comes to chemistry, one of the most fundamental skills you need to master is naming compounds. Whether you’re a student or a professional, being able to accurately identify and name compounds is crucial for communication and understanding in the field. In this guide, we’ll walk you through the basics of naming compounds and provide you with a worksheet to help you practice and reinforce your knowledge.
Understanding the Basics of Naming Compounds
Before we dive into the specifics of naming compounds, let’s cover some basic concepts:
- Atoms: The building blocks of matter, atoms are the smallest units of a chemical element.
- Molecules: A group of atoms bonded together, molecules are the smallest units of a chemical compound.
- Compounds: A substance formed by the chemical bonding of two or more different elements, compounds can be either molecular (covalent) or ionic.
Naming Molecular Compounds
Molecular compounds are formed when two or more nonmetals bond together. Here are the steps to follow when naming molecular compounds:
- Identify the elements: Determine the elements present in the compound and their respective numbers.
- Determine the prefixes: Use the prefixes listed below to indicate the number of atoms of each element:
- Mono- (1)
- Di- (2)
- Tri- (3)
- Tetra- (4)
- Penta- (5)
- Hexa- (6)
- Hepta- (7)
- Octa- (8)
- Nona- (9)
- Deca- (10)
- Combine the prefixes: Combine the prefixes with the root words of the elements to form the compound name.
Example: CO2 (carbon dioxide)
- Identify the elements: carbon © and oxygen (O)
- Determine the prefixes: mono- (1) for carbon and di- (2) for oxygen
- Combine the prefixes: monoxide
Worksheet Exercise: Try naming the following molecular compounds:

| Compound | Formula |
|---|---|
| 1 | CH4 |
| 2 | N2O |
| 3 | CO |
| 4 | H2S |
Naming Ionic Compounds
Ionic compounds are formed when a metal and a nonmetal bond together. Here are the steps to follow when naming ionic compounds:
- Identify the cation: Determine the metal present in the compound and its charge.
- Identify the anion: Determine the nonmetal present in the compound and its charge.
- Combine the cation and anion: Combine the names of the cation and anion to form the compound name.
Example: NaCl (sodium chloride)
- Identify the cation: sodium (Na+)
- Identify the anion: chloride (Cl-)
- Combine the cation and anion: sodium chloride
Worksheet Exercise: Try naming the following ionic compounds:
| Compound | Formula |
|---|---|
| 1 | CaO |
| 2 | MgCl2 |
| 3 | Fe2O3 |
| 4 | CuSO4 |
Naming Acids and Bases
Acids and bases are special types of compounds that have unique naming conventions. Here are the steps to follow when naming acids and bases:
- Acids: Use the prefix “hydro-” followed by the root word of the anion and the suffix “-ic” or “-ous”.
- Bases: Use the name of the cation followed by the suffix “-hydroxide”.
Example: HCl (hydrochloric acid)
- Identify the anion: chloride (Cl-)
- Use the prefix “hydro-” and the suffix “-ic”: hydrochloric acid
Worksheet Exercise: Try naming the following acids and bases:
| Compound | Formula |
|---|---|
| 1 | H2SO4 |
| 2 | NaOH |
| 3 | HNO3 |
| 4 | KOH |
Conclusion
Naming compounds is a fundamental skill in chemistry that requires practice and patience. With this guide and worksheet, you’ll be well on your way to mastering the art of naming compounds. Remember to identify the elements, determine the prefixes, and combine the prefixes with the root words to form the compound name. Happy practicing!
What is the difference between a molecular compound and an ionic compound?
+A molecular compound is formed when two or more nonmetals bond together, while an ionic compound is formed when a metal and a nonmetal bond together.
How do I determine the prefixes for molecular compounds?
+Use the prefixes listed in the guide to indicate the number of atoms of each element: mono- (1), di- (2), tri- (3), and so on.
What is the difference between an acid and a base?
+An acid is a compound that donates a proton (H+), while a base is a compound that accepts a proton (H+).