6 Ways to Master Mixtures, Elements, and Compounds
Chemistry is a fundamental subject that deals with the study of matter, its properties, and the changes it undergoes. One of the crucial aspects of chemistry is understanding the differences between mixtures, elements, and compounds. These three terms are often used interchangeably, but they have distinct meanings. In this article, we will explore six ways to master mixtures, elements, and compounds, helping you to understand the concepts better.
Understanding the Basics
Before diving into the differences between mixtures, elements, and compounds, it’s essential to understand the basic definitions.
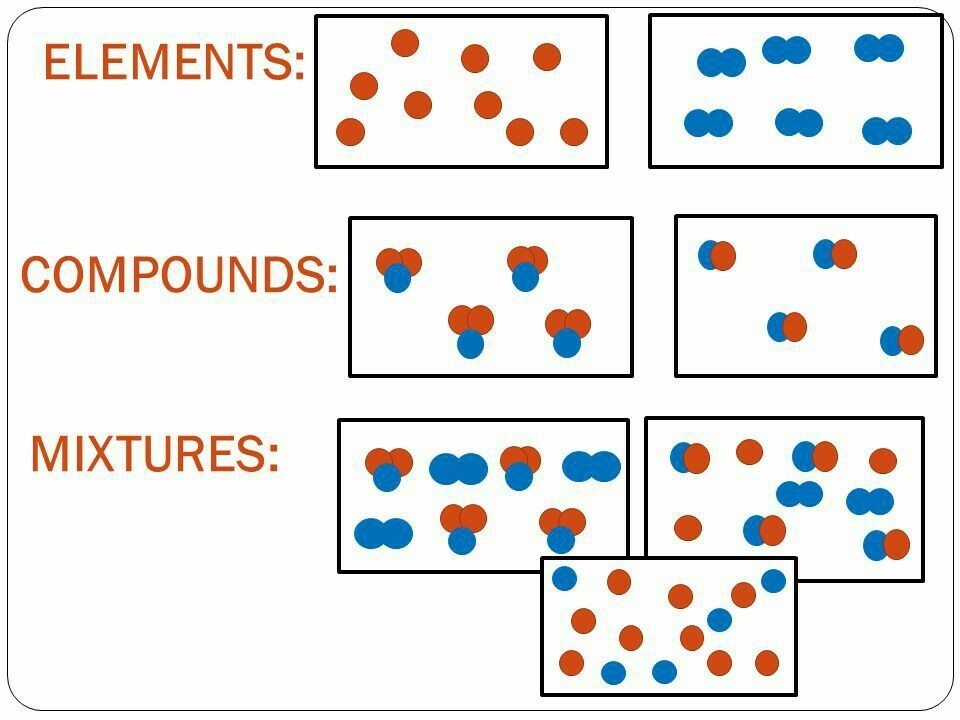
- Mixtures: A mixture is a physical blend of two or more substances, where each substance retains its chemical properties. Examples include air, seawater, and blood.
- Elements: An element is a pure substance consisting of only one type of atom, distinguished by its atomic number. Examples include hydrogen, oxygen, and carbon.
- Compounds: A compound is a substance formed when two or more different elements are chemically bonded together. Examples include water (H2O), carbon dioxide (CO2), and salt (NaCl).
1. Learn the Symbols and Notations
To master mixtures, elements, and compounds, it’s crucial to learn the symbols and notations used to represent them. Elements are represented by their atomic symbols, which are usually derived from the element’s name. For example, hydrogen is represented by the symbol H, and oxygen is represented by the symbol O.
Compounds, on the other hand, are represented by their chemical formulas, which indicate the number of atoms of each element present in the compound. For example, the chemical formula for water is H2O, indicating that one molecule of water consists of two hydrogen atoms and one oxygen atom.
Mixtures are not represented by a specific symbol or notation, but rather by the combination of the symbols of the individual substances present in the mixture.
2. Understand the Physical and Chemical Properties
Another way to master mixtures, elements, and compounds is to understand their physical and chemical properties. Elements have unique physical and chemical properties, such as melting and boiling points, density, and reactivity.
Compounds, on the other hand, have properties that are different from those of their individual elements. For example, the melting point of water is 0°C, which is different from the melting points of hydrogen and oxygen.
Mixtures also have physical and chemical properties, but they can vary depending on the proportion of the individual substances present in the mixture.
3. Learn to Identify and Separate Mixtures
Identifying and separating mixtures is an essential skill in mastering mixtures, elements, and compounds. There are several methods to separate mixtures, including:
- Filtration: This involves passing the mixture through a filter to separate the solid particles from the liquid.
- Distillation: This involves heating the mixture to separate the components based on their boiling points.
- Chromatography: This involves separating the components of a mixture based on their affinity for a stationary phase.
4. Understand Chemical Reactions
Chemical reactions involve the transformation of one substance into another. Understanding chemical reactions is crucial in mastering mixtures, elements, and compounds. There are several types of chemical reactions, including:
- Synthesis reactions: These involve the combination of two or more substances to form a new compound.
- Decomposition reactions: These involve the breakdown of a compound into its individual elements.
- Replacement reactions: These involve the replacement of one element with another in a compound.
5. Practice with Examples and Exercises
Practicing with examples and exercises is an effective way to master mixtures, elements, and compounds. Try solving problems and exercises that involve identifying and separating mixtures, writing chemical formulas, and balancing chemical equations.
6. Use Visual Aids and Diagrams
Using visual aids and diagrams can help to reinforce your understanding of mixtures, elements, and compounds. Try creating diagrams to illustrate the structure of compounds, or use flowcharts to illustrate the steps involved in separating mixtures.
💡 Note: Visual aids and diagrams can be especially helpful when trying to understand complex chemical reactions and structures.
Conclusion
Mastering mixtures, elements, and compounds requires a combination of understanding the basics, learning symbols and notations, understanding physical and chemical properties, identifying and separating mixtures, understanding chemical reactions, practicing with examples and exercises, and using visual aids and diagrams. By following these six ways, you’ll be well on your way to becoming proficient in chemistry and understanding the differences between mixtures, elements, and compounds.
Frequently Asked Questions
What is the difference between a mixture and a compound?
+A mixture is a physical blend of two or more substances, where each substance retains its chemical properties. A compound, on the other hand, is a substance formed when two or more different elements are chemically bonded together.
How can I separate a mixture of sand and water?
+One way to separate a mixture of sand and water is through filtration. You can pass the mixture through a filter, such as a coffee filter or a paper towel, to separate the sand from the water.
What is the chemical formula for carbon dioxide?
+The chemical formula for carbon dioxide is CO2.