Isotopes Worksheets With Answers for Easy Learning
Understanding Isotopes: A Comprehensive Guide for Students
Isotopes are a fundamental concept in chemistry, and understanding them is crucial for students to grasp various chemical reactions and processes. In this article, we will provide a detailed explanation of isotopes, their types, and importance, along with some practice worksheets and answers to help students learn easily.
What are Isotopes?
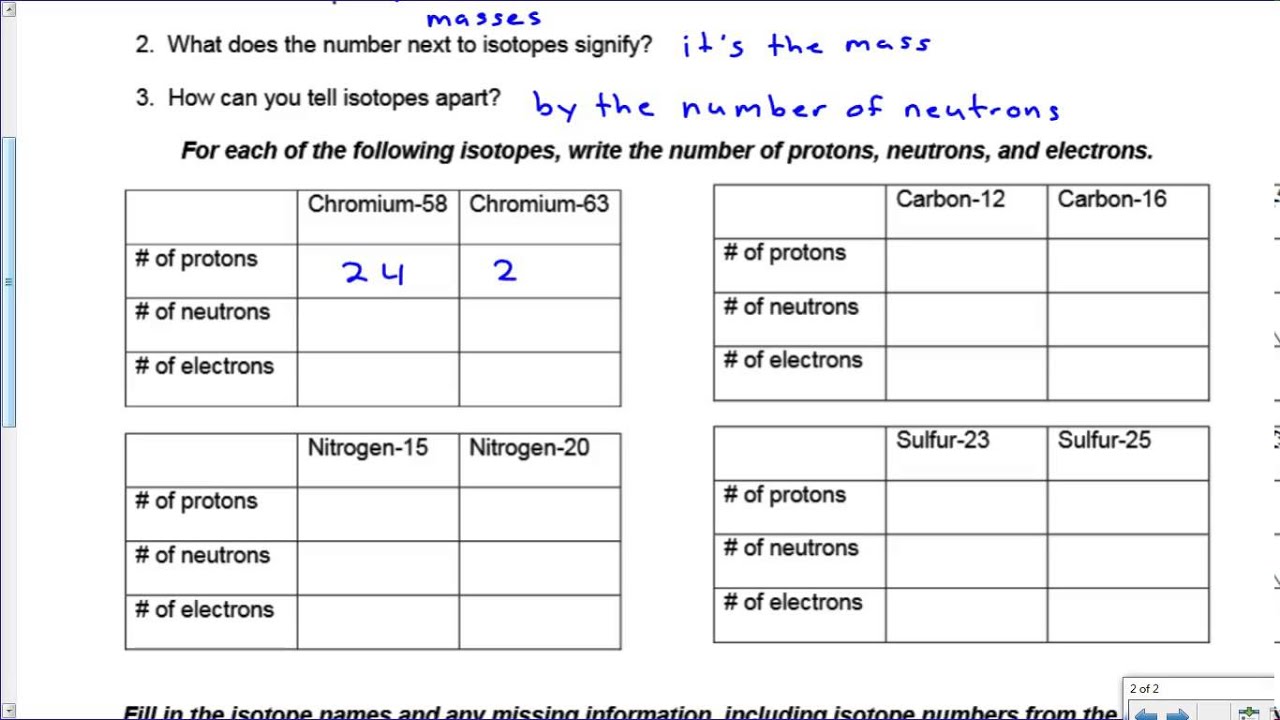
Isotopes are atoms of the same chemical element that have the same number of protons in their atomic nuclei but differ in the number of neutrons. This difference in neutrons leads to variations in the atomic mass of isotopes. For example, carbon-12, carbon-13, and carbon-14 are three isotopes of the element carbon, with different numbers of neutrons.
Types of Isotopes
There are two main types of isotopes: stable and radioactive.
- Stable Isotopes: These isotopes do not undergo radioactive decay and remain stable over time. Examples include carbon-12 and oxygen-16.
- Radioactive Isotopes: These isotopes undergo radioactive decay, emitting radiation to become stable. Examples include carbon-14 and uranium-238.
Importance of Isotopes
Isotopes have numerous applications in various fields, including:
- Medicine: Radioactive isotopes are used in medical imaging and cancer treatment.
- Environmental Science: Stable isotopes help track climate change and study ecosystems.
- Food Industry: Isotopes are used to detect food adulteration and ensure food safety.
Isotopes Worksheets with Answers
To help students practice and reinforce their understanding of isotopes, we have created some worksheets with answers.
Worksheet 1: Multiple Choice Questions
- What is the primary difference between isotopes of the same element?
- A) Number of protons
- B) Number of neutrons
- C) Atomic mass
- D) Electron configuration
Answer: B) Number of neutrons
- Which of the following is an example of a stable isotope?
- A) Carbon-14
- B) Oxygen-16
- C) Uranium-238
- D) Radium-226
Answer: B) Oxygen-16
Worksheet 2: Short Answer Questions
- Describe the difference between stable and radioactive isotopes.
Answer: Stable isotopes do not undergo radioactive decay, while radioactive isotopes emit radiation to become stable.
- What is one application of isotopes in medicine?
Answer: Radioactive isotopes are used in medical imaging and cancer treatment.
Worksheet 3: Fill-in-the-Blank Questions
- The number of protons in an atom’s nucleus determines its _______________________.
Answer: Atomic number
- Isotopes with the same number of protons but different numbers of neutrons have different _______________________.
Answer: Atomic masses
📝 Note: These worksheets are meant to provide a starting point for students to practice and learn about isotopes. Teachers and educators can modify and expand on these worksheets to suit their teaching needs.
Conclusion
In conclusion, isotopes are a vital concept in chemistry, and understanding them is essential for students to grasp various chemical reactions and processes. By practicing with worksheets and learning about the different types and applications of isotopes, students can develop a deeper understanding of this fundamental concept.
What is the primary difference between isotopes of the same element?
+
The primary difference between isotopes of the same element is the number of neutrons in their atomic nuclei.
What is one application of isotopes in medicine?
+
Radioactive isotopes are used in medical imaging and cancer treatment.
What is the difference between stable and radioactive isotopes?
+
Stable isotopes do not undergo radioactive decay, while radioactive isotopes emit radiation to become stable.