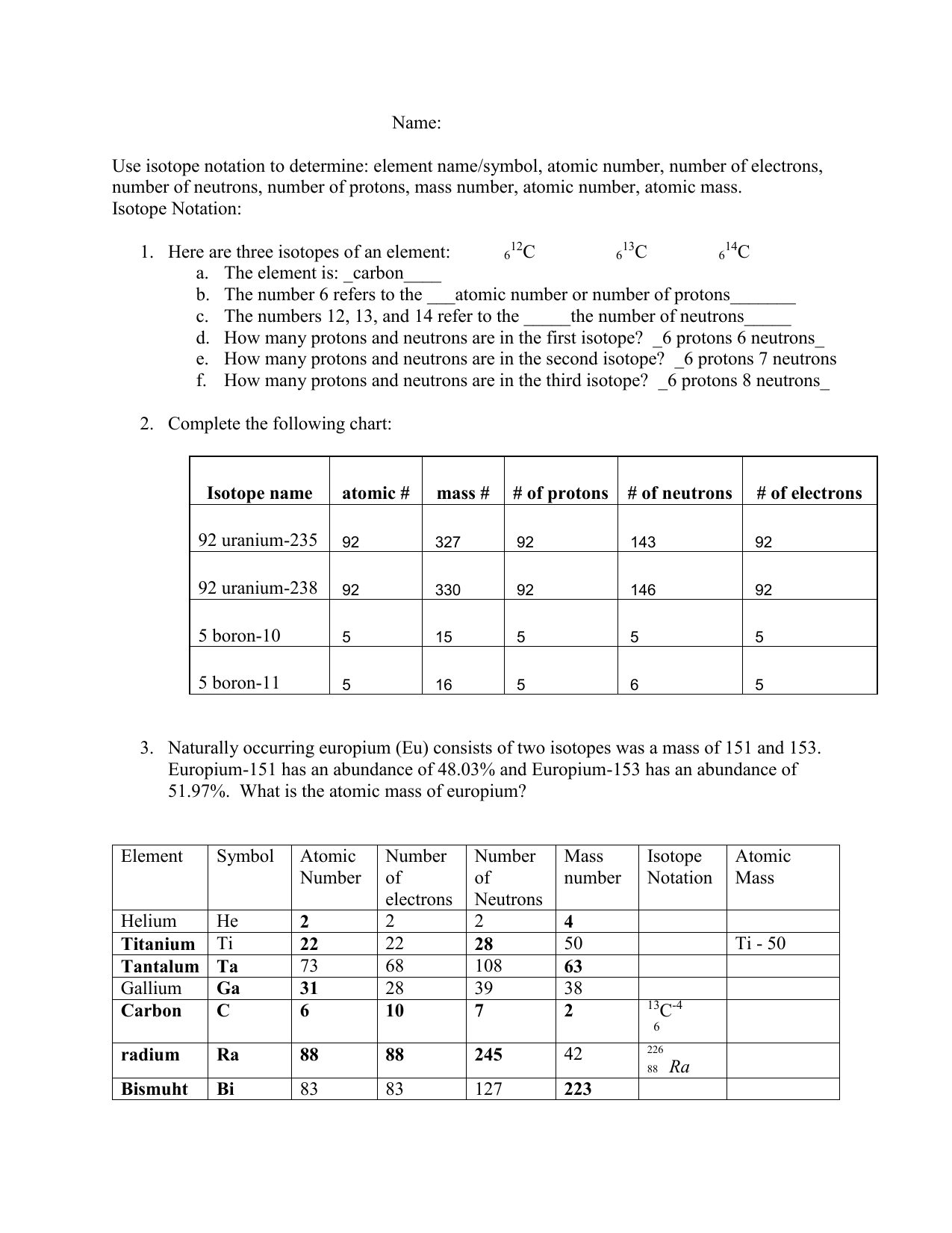
Isotope Worksheet Answers
Understanding Isotopes: A Comprehensive Guide
Atoms are the building blocks of matter, and they consist of protons, neutrons, and electrons. While the number of protons in an atom’s nucleus determines the element of an atom, the number of neutrons can vary, leading to different isotopes of the same element. In this article, we will delve into the world of isotopes, exploring what they are, how they are formed, and their significance in various fields.
What are Isotopes?
Isotopes are atoms of the same chemical element that have the same number of protons but differ in the number of neutrons in their nuclei. This variation in neutron number affects the atomic mass of the isotopes, making them distinct from one another. For instance, carbon-12, carbon-13, and carbon-14 are all isotopes of the element carbon, with 6 protons in their atomic nuclei but varying numbers of neutrons (6, 7, and 8, respectively).
Types of Isotopes
There are two main types of isotopes: stable and radioactive.
- Stable Isotopes: These isotopes have a stable nucleus and do not undergo radioactive decay. Examples of stable isotopes include carbon-12 and oxygen-16.
- Radioactive Isotopes: These isotopes have an unstable nucleus and undergo radioactive decay, emitting radiation to become more stable. Examples of radioactive isotopes include carbon-14 and uranium-238.
Formation of Isotopes
Isotopes are formed through various natural and artificial processes.
- Natural Processes: Isotopes can be formed through natural processes such as nuclear reactions in stars, cosmic ray bombardment, and radioactive decay.
- Artificial Processes: Isotopes can be created artificially through particle bombardment, nuclear reactions, and other laboratory techniques.
Significance of Isotopes
Isotopes have numerous applications in various fields, including:
- Geology: Isotopes are used to determine the age of rocks and fossils, helping us understand the Earth’s history.
- Medicine: Radioactive isotopes are used in medical imaging and cancer treatment.
- Environmental Science: Isotopes are used to study climate change, track ocean currents, and monitor water cycles.
- Food Safety: Isotopes are used to detect food adulteration and contamination.
Isotope Fractionation
Isotope fractionation is the process by which isotopes are separated based on their mass difference. This process occurs in various natural and artificial systems, including:
- Biological Systems: Isotope fractionation occurs in living organisms, influencing the isotopic composition of biological tissues.
- Environmental Systems: Isotope fractionation occurs in environmental systems, affecting the isotopic composition of water, air, and soil.
Isotope Analysis Techniques
Several techniques are used to analyze isotopes, including:
- Mass Spectrometry: This technique measures the mass-to-charge ratio of ions, allowing for the identification and quantification of isotopes.
- Gas Chromatography: This technique separates and analyzes gases based on their boiling points and affinity for a stationary phase.
- Spectroscopy: This technique measures the interaction between light and matter, providing information on the isotopic composition of a sample.
Conclusion
Isotopes are an essential aspect of modern science, with applications in various fields. Understanding isotopes and their properties is crucial for advancing our knowledge of the natural world and addressing global challenges. By recognizing the significance of isotopes, we can harness their potential to improve our daily lives and create a more sustainable future.
What is the difference between an isotope and an element?
+An element is a substance that consists of atoms with the same number of protons in the atomic nucleus, whereas an isotope is a variant of an element that has the same number of protons but a different number of neutrons.
How are isotopes used in medicine?
+Isotopes are used in medicine for diagnostic and therapeutic purposes. Radioactive isotopes are used in imaging techniques such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT), while stable isotopes are used to diagnose diseases such as diabetes and cancer.
What is the significance of isotopes in environmental science?
+Isotopes play a crucial role in environmental science, as they are used to study climate change, track ocean currents, and monitor water cycles. Isotopes are also used to detect food adulteration and contamination, ensuring food safety.
Related Terms:
- Isotope Practice Worksheet answers PDF
- Isotope Practice Worksheet pdf
- Chemistry isotopes Worksheet