Mastering Ionic Bonding with Worksheets
Understanding Ionic Bonding
Ionic bonding is a type of chemical bonding that involves the transfer of electrons between atoms, resulting in the formation of ions with opposite charges. These ions are then attracted to each other, forming a strong electrostatic bond. This type of bonding is commonly seen in compounds formed between metals and nonmetals.
The Formation of Ionic Bonds
The formation of ionic bonds can be explained by the following steps:
- Step 1: Electron Transfer: A metal atom loses one or more electrons to form a positively charged ion, known as a cation. This process is called oxidation.
- Step 2: Electron Gain: A nonmetal atom gains one or more electrons to form a negatively charged ion, known as an anion. This process is called reduction.
- Step 3: Electrostatic Attraction: The oppositely charged cation and anion are attracted to each other, resulting in the formation of a strong electrostatic bond.
🔍 Note: The formation of ionic bonds is a result of the transfer of electrons, not the sharing of electrons, which is characteristic of covalent bonds.
Characteristics of Ionic Compounds
Ionic compounds have several distinct characteristics:
- High Melting and Boiling Points: Ionic compounds have high melting and boiling points due to the strong electrostatic forces between the ions.
- Hard and Brittle: Ionic compounds are typically hard and brittle, as the ions are arranged in a rigid and regular lattice structure.
- Conduct Electricity: Ionic compounds can conduct electricity when dissolved in water or melted, as the ions are free to move and carry charge.
Examples of Ionic Compounds
Some examples of ionic compounds include:
- Sodium Chloride (NaCl): Also known as table salt, this compound is formed by the transfer of an electron from sodium to chlorine.
- Calcium Carbonate (CaCO3): This compound is formed by the transfer of electrons from calcium to carbonate.
- Potassium Nitrate (KNO3): This compound is formed by the transfer of electrons from potassium to nitrate.
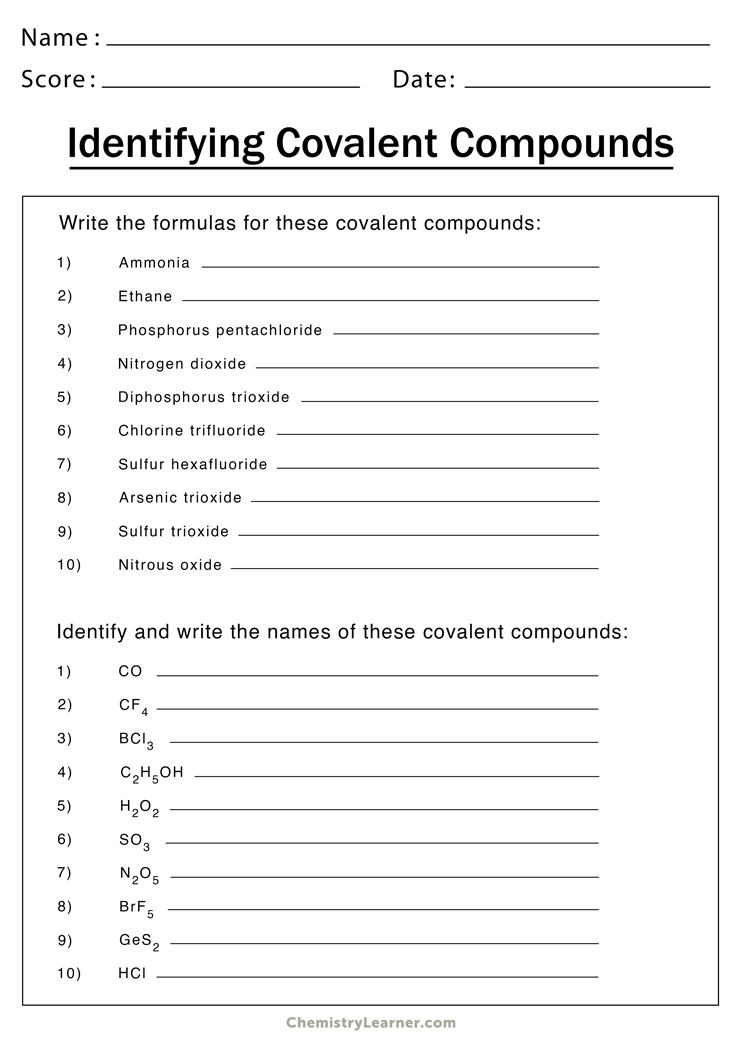
Worksheets for Ionic Bonding
Here are some worksheets to help you practice and reinforce your understanding of ionic bonding:
Worksheet 1: Electron Transfer
| Metal | Nonmetal | Cation | Anion |
|---|---|---|---|
| Sodium (Na) | Chlorine (Cl) | Na+ | Cl- |
| Calcium (Ca) | Oxygen (O) | Ca2+ | O2- |
| Potassium (K) | Nitrogen (N) | K+ | N3- |
Complete the table by writing the correct cation and anion for each combination of metal and nonmetal.
Worksheet 2: Ionic Compound Formation
| Cation | Anion | Ionic Compound |
|---|---|---|
| Na+ | Cl- | |
| Ca2+ | O2- | |
| K+ | N3- |
Complete the table by writing the correct ionic compound for each combination of cation and anion.
Worksheet 3: Characteristics of Ionic Compounds
| Characteristic | Explanation |
|---|---|
| High melting and boiling points | |
| Hard and brittle | |
| Conduct electricity |
Complete the table by writing a brief explanation for each characteristic of ionic compounds.
📝 Note: These worksheets are designed to help you practice and reinforce your understanding of ionic bonding. Be sure to review the concepts and complete the worksheets carefully.
The concept of ionic bonding is a fundamental aspect of chemistry, and understanding how ions interact with each other is crucial for understanding many chemical reactions and processes. By mastering ionic bonding, you will be better equipped to tackle more complex topics in chemistry and other sciences.
What is the main difference between ionic and covalent bonds?
+
The main difference between ionic and covalent bonds is the way in which the electrons are distributed between the atoms. In ionic bonds, electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges. In covalent bonds, electrons are shared between the atoms.
What are some examples of ionic compounds?
+
Some examples of ionic compounds include sodium chloride (NaCl), calcium carbonate (CaCO3), and potassium nitrate (KNO3).
Why do ionic compounds have high melting and boiling points?
+
Ionic compounds have high melting and boiling points due to the strong electrostatic forces between the ions.



