7 Ways to Master Intermolecular Forces
Understanding Intermolecular Forces: A Key to Mastering Chemistry
Intermolecular forces are a crucial aspect of chemistry, playing a vital role in determining the properties and behavior of molecules. These forces are responsible for the physical state of a substance, its solubility, and its ability to conduct heat and electricity. Mastering intermolecular forces is essential for any chemistry student or professional, as it helps to understand the underlying principles of chemical reactions and interactions.
What are Intermolecular Forces?
Intermolecular forces are the attractive and repulsive forces between molecules. They are weaker than the covalent bonds that hold atoms together within a molecule, but stronger than the gravitational forces that act between planets. There are several types of intermolecular forces, including:
- Van der Waals forces: These are the weakest intermolecular forces, resulting from temporary dipoles in non-polar molecules.
- Dipole-dipole forces: These forces occur between polar molecules, where the positive end of one molecule is attracted to the negative end of another.
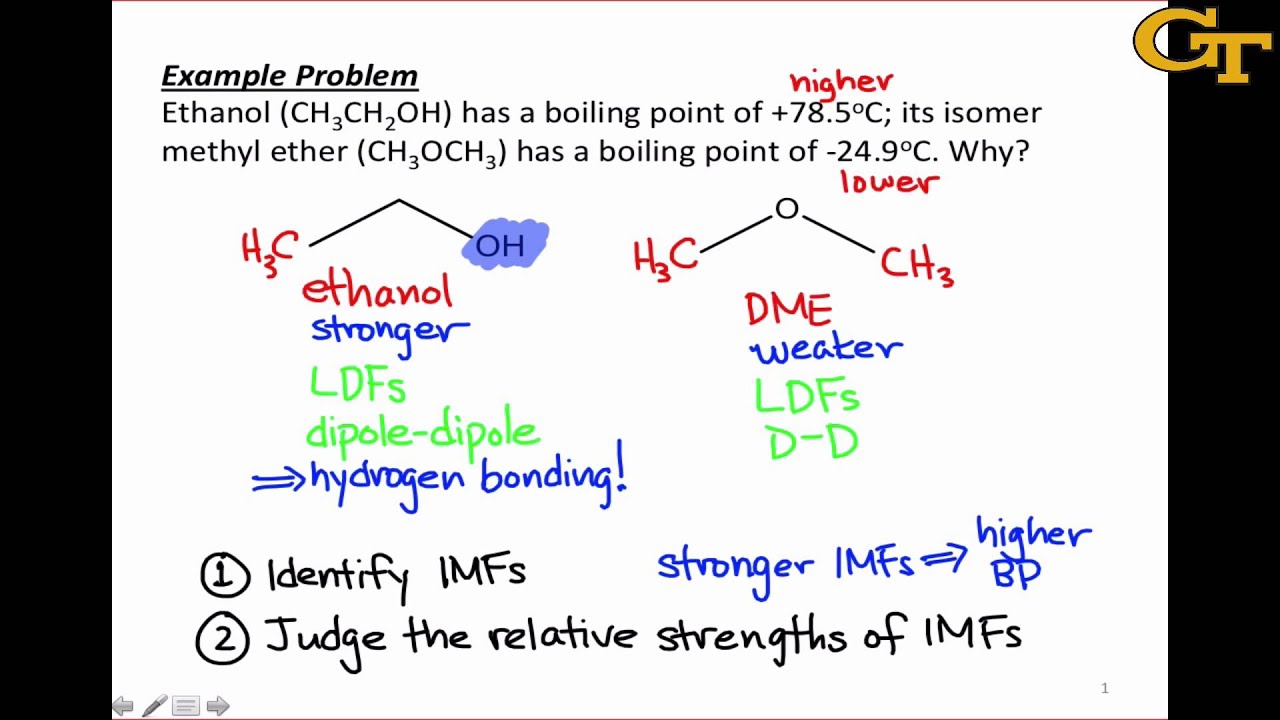
- Hydrogen bonding: This is a special type of dipole-dipole force that occurs between molecules with a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine).
- Ion-dipole forces: These forces occur between ions and polar molecules.
7 Ways to Master Intermolecular Forces
Mastering intermolecular forces requires a deep understanding of their nature and properties. Here are 7 ways to help you achieve mastery:
1. Understand the Different Types of Intermolecular Forces
Each type of intermolecular force has its own unique characteristics and properties. Understanding these differences is crucial to predicting the behavior of molecules.
- Van der Waals forces: These forces are responsible for the physical state of a substance (solid, liquid, or gas). They are also responsible for the viscosity and surface tension of a liquid.
- Dipole-dipole forces: These forces are responsible for the solubility of polar substances in polar solvents.
- Hydrogen bonding: This force is responsible for the high boiling points of substances such as water and ammonia.
- Ion-dipole forces: These forces are responsible for the solubility of ionic substances in polar solvents.
2. Learn to Identify Polar and Non-Polar Molecules
Identifying polar and non-polar molecules is essential to predicting the type of intermolecular force that will occur between them. Polar molecules have a permanent dipole moment, while non-polar molecules do not.
- Polar molecules: These molecules have a permanent dipole moment, resulting from a difference in electronegativity between the atoms in the molecule.
- Non-polar molecules: These molecules do not have a permanent dipole moment, resulting from a symmetrical distribution of electrons around the molecule.
3. Use Lewis Structures to Visualize Intermolecular Forces
Lewis structures are a powerful tool for visualizing the shape and polarity of molecules. They can help you identify the type of intermolecular force that will occur between molecules.
- Draw the Lewis structure of the molecule: Use the Lewis structure to identify the polarity of the molecule and the type of intermolecular force that will occur.
- Identify the electronegative atoms: Electronegative atoms such as oxygen, nitrogen, and fluorine can create a permanent dipole moment in a molecule.
4. Understand the Relationship Between Intermolecular Forces and Physical Properties
Intermolecular forces are responsible for many of the physical properties of a substance, including its boiling point, melting point, viscosity, and surface tension.
- Boiling point: The boiling point of a substance is directly related to the strength of the intermolecular forces between its molecules.
- Melting point: The melting point of a substance is also related to the strength of the intermolecular forces between its molecules.
- Viscosity: The viscosity of a liquid is related to the strength of the intermolecular forces between its molecules.
- Surface tension: The surface tension of a liquid is related to the strength of the intermolecular forces between its molecules.
5. Practice, Practice, Practice
Practice is essential to mastering intermolecular forces. Try solving problems and answering questions to test your understanding.
- Solve problems: Try solving problems that involve identifying the type of intermolecular force that will occur between molecules.
- Answer questions: Try answering questions that test your understanding of intermolecular forces and their properties.
6. Watch Video Tutorials and Online Lectures
Video tutorials and online lectures can provide a wealth of information on intermolecular forces and their properties.
- Watch video tutorials: Video tutorials can provide a visual explanation of intermolecular forces and their properties.
- Watch online lectures: Online lectures can provide a more in-depth explanation of intermolecular forces and their properties.
7. Join Online Communities and Discussion Forums
Joining online communities and discussion forums can provide a wealth of information and resources on intermolecular forces.
- Join online communities: Join online communities such as Reddit’s r/chemistry community to ask questions and discuss topics related to intermolecular forces.
- Participate in discussion forums: Participate in discussion forums to ask questions and discuss topics related to intermolecular forces.
📝 Note: Mastering intermolecular forces takes time and practice. Be patient and persistent, and you will eventually develop a deep understanding of these forces and their properties.
In conclusion, mastering intermolecular forces is essential for any chemistry student or professional. By understanding the different types of intermolecular forces, learning to identify polar and non-polar molecules, using Lewis structures to visualize intermolecular forces, understanding the relationship between intermolecular forces and physical properties, practicing, watching video tutorials and online lectures, and joining online communities and discussion forums, you can develop a deep understanding of these forces and their properties.
What are the different types of intermolecular forces?
+There are several types of intermolecular forces, including Van der Waals forces, dipole-dipole forces, hydrogen bonding, and ion-dipole forces.
How do I identify polar and non-polar molecules?
+Polar molecules have a permanent dipole moment, resulting from a difference in electronegativity between the atoms in the molecule. Non-polar molecules do not have a permanent dipole moment, resulting from a symmetrical distribution of electrons around the molecule.
What is the relationship between intermolecular forces and physical properties?
+Intermolecular forces are responsible for many of the physical properties of a substance, including its boiling point, melting point, viscosity, and surface tension.
Related Terms:
- Intermolecular forces Worksheet answer Key
- Intermolecular forces Worksheet 2 Answers
- Intermolecular forces Worksheet dr slotsky
- Intermolecular forces Webquest
- Intermolecular forces powerpoint
- Intermolecular forces PDF