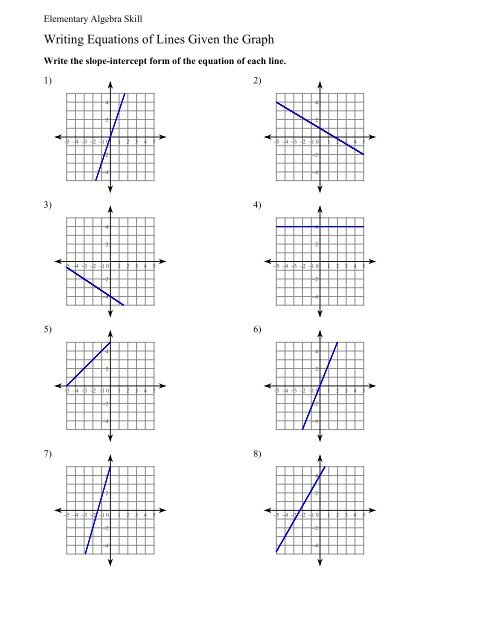
Hard Balancing Equations Worksheet with Answers

Mastering Chemical Equations: A Comprehensive Guide to Balancing
Balancing chemical equations is a fundamental skill in chemistry that can be challenging to master, especially for those who are new to the subject. However, with practice and persistence, anyone can become proficient in balancing equations. In this article, we will provide a step-by-step guide on how to balance chemical equations, along with some examples and a worksheet with answers.
Why is Balancing Equations Important?
Balancing chemical equations is crucial in chemistry because it allows us to:
- Predict the amounts of reactants and products: By balancing an equation, we can determine the exact amounts of reactants required to produce a certain amount of product.
- Determine the limiting reactant: Balancing equations helps us identify the reactant that will run out first, which is essential in determining the maximum amount of product that can be formed.
- Calculate the yield of a reaction: By balancing an equation, we can calculate the percentage yield of a reaction, which is the ratio of the actual amount of product formed to the theoretical amount.
Step-by-Step Guide to Balancing Equations
Balancing chemical equations involves making sure that the number of atoms of each element is the same on both the reactant and product sides. Here’s a step-by-step guide on how to balance equations:
- Write the unbalanced equation: Start by writing the unbalanced equation with the reactants on the left and the products on the right.
- Count the atoms: Count the number of atoms of each element on both the reactant and product sides.
- Identify the elements that need balancing: Identify the elements that have a different number of atoms on the reactant and product sides.
- Add coefficients: Add coefficients (numbers in front of the formulas of reactants or products) to balance the elements that need balancing.
- Check the balance: Check the balance of the equation by counting the atoms again.
Examples of Balancing Equations
Here are some examples of balancing equations:
Example 1:
Unbalanced equation: Ca + HCl → CaCl2 + H2
Balanced equation: Ca + 2HCl → CaCl2 + H2
Example 2:
Unbalanced equation: Fe + O2 → Fe2O3
Balanced equation: 4Fe + 3O2 → 2Fe2O3
Hard Balancing Equations Worksheet with Answers
Here’s a worksheet with 10 balancing equations for you to practice. The answers are provided at the end.
- Unbalanced equation:
Al + CuSO4 → Al2(SO4)3 + Cu - Unbalanced equation:
NaOH + HCl → NaCl + H2O - Unbalanced equation:
CaCO3 → CaO + CO2 - Unbalanced equation:
Fe + HCl → FeCl2 + H2 - Unbalanced equation:
Na + H2O → NaOH + H2 - Unbalanced equation:
Cu + HNO3 → Cu(NO3)2 + NO2 + H2O - Unbalanced equation:
Al + HCl → AlCl3 + H2 - Unbalanced equation:
Zn + HCl → ZnCl2 + H2 - Unbalanced equation:
Na + O2 → Na2O - Unbalanced equation:
Fe + O2 → Fe2O3
Answers:
2Al + 3CuSO4 → Al2(SO4)3 + 3CuNaOH + HCl → NaCl + H2OCaCO3 → CaO + CO2Fe + 2HCl → FeCl2 + H22Na + 2H2O → 2NaOH + H2Cu + 4HNO3 → Cu(NO3)2 + 2NO2 + 2H2O2Al + 6HCl → 2AlCl3 + 3H2Zn + 2HCl → ZnCl2 + H24Na + O2 → 2Na2O4Fe + 3O2 → 2Fe2O3
📝 Note: Remember to count the atoms carefully and add coefficients to balance the elements that need balancing.
By practicing with these examples and worksheet, you’ll become proficient in balancing chemical equations in no time!
What is the purpose of balancing chemical equations?
+
The purpose of balancing chemical equations is to ensure that the number of atoms of each element is the same on both the reactant and product sides, which allows us to predict the amounts of reactants and products, determine the limiting reactant, and calculate the yield of a reaction.
How do I balance a chemical equation?
+
To balance a chemical equation, start by writing the unbalanced equation, count the atoms, identify the elements that need balancing, add coefficients, and check the balance.
What is a coefficient in a chemical equation?
+
A coefficient is a number in front of the formula of a reactant or product that indicates the number of molecules of that substance involved in the reaction.