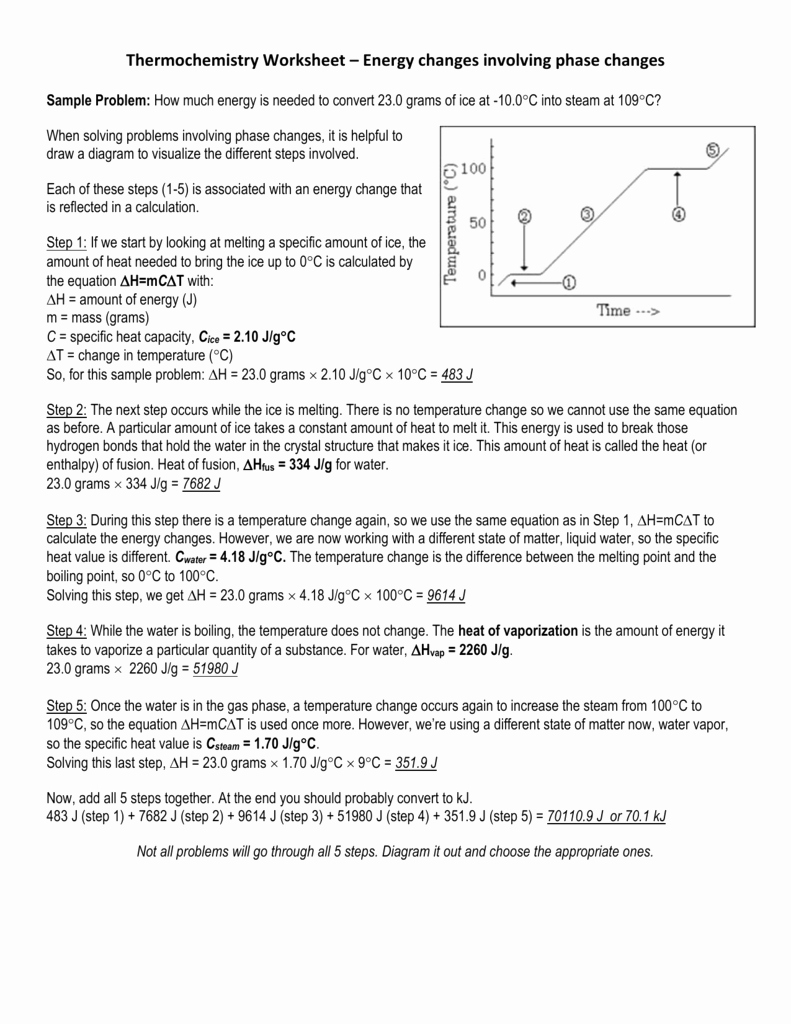
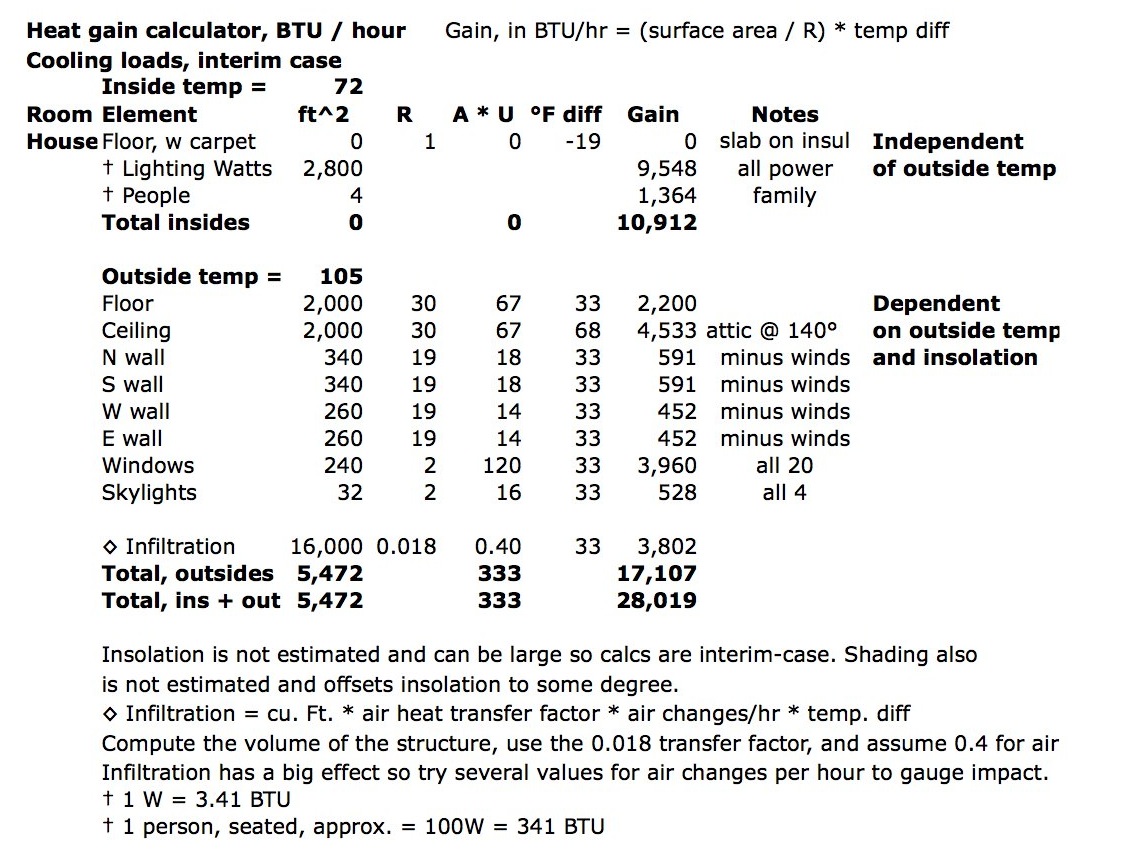
5 Ways to Master Energy Transformation Worksheet Answers

Mastering energy transformation is a crucial aspect of understanding how energy is converted from one form to another. It is a fundamental concept in physics and engineering, and having a solid grasp of it can help you solve complex problems and optimize systems. In this article, we will provide you with 5 ways to master energy transformation, along with a worksheet to help you practice and reinforce your understanding.
Understanding Energy Transformation
Before we dive into the ways to master energy transformation, let’s first define what it is. Energy transformation is the process of converting energy from one form to another. For example, a car engine converts chemical energy from gasoline into mechanical energy, which propels the car forward. Energy transformation is an essential concept in physics and engineering, as it helps us understand how energy is converted and utilized in various systems.
Way 1: Understand the Different Types of Energy
To master energy transformation, you need to understand the different types of energy. There are several types of energy, including:
- Kinetic Energy: the energy of motion
- Potential Energy: stored energy that has the potential to do work
- Thermal Energy: the energy of heat
- Electrical Energy: the energy of moving charges
- Chemical Energy: the energy stored in chemical bonds
- Nuclear Energy: the energy stored in the nucleus of an atom
Each type of energy has its unique characteristics and can be converted into other forms of energy. Understanding the different types of energy is crucial in mastering energy transformation.
Way 2: Learn the Laws of Thermodynamics
The laws of thermodynamics are fundamental principles that govern energy transformation. There are four laws of thermodynamics:
- Zeroth Law of Thermodynamics: if two systems are in thermal equilibrium with a third system, then they are also in thermal equilibrium with each other
- First Law of Thermodynamics: energy cannot be created or destroyed, only converted from one form to another
- Second Law of Thermodynamics: the total entropy of an isolated system always increases over time
- Third Law of Thermodynamics: as the temperature of a system approaches absolute zero, the entropy of the system approaches a minimum value
Understanding the laws of thermodynamics is essential in mastering energy transformation, as they provide a framework for understanding how energy is converted and utilized.
Way 3: Practice Energy Transformation Problems
To master energy transformation, you need to practice solving problems that involve energy conversion. Here are a few examples:
- A car engine converts chemical energy from gasoline into mechanical energy. If the engine has an efficiency of 20%, how much mechanical energy is produced from 100 J of chemical energy?
- A solar panel converts sunlight into electrical energy. If the panel has an area of 10 m^2 and receives 500 W/m^2 of sunlight, how much electrical energy is produced in 1 hour?
Practicing energy transformation problems will help you develop a deeper understanding of how energy is converted and utilized.
Way 4: Use Energy Transformation Diagrams
Energy transformation diagrams are a useful tool for visualizing how energy is converted from one form to another. A typical energy transformation diagram shows the different forms of energy and how they are converted into each other.
Here is an example of an energy transformation diagram:

| Energy Form | Conversion | Energy Form |
|---|---|---|
| Chemical Energy | Combustion | Thermal Energy |
| Thermal Energy | Heat Engine | Mechanical Energy |
| Mechanical Energy | Generator | Electrical Energy |
Using energy transformation diagrams will help you visualize how energy is converted and utilized in various systems.
Way 5: Apply Energy Transformation to Real-World Systems
To master energy transformation, you need to apply it to real-world systems. Here are a few examples:
- A power plant converts chemical energy from coal into electrical energy. How much electrical energy is produced from 1000 kg of coal?
- A car engine converts chemical energy from gasoline into mechanical energy. How much mechanical energy is produced from 100 L of gasoline?
Applying energy transformation to real-world systems will help you develop a deeper understanding of how energy is converted and utilized in various systems.
Conclusion
Mastering energy transformation is a crucial aspect of understanding how energy is converted from one form to another. By understanding the different types of energy, learning the laws of thermodynamics, practicing energy transformation problems, using energy transformation diagrams, and applying energy transformation to real-world systems, you can develop a deep understanding of energy transformation. Remember to practice and reinforce your understanding with worksheet answers.
Worksheet Answers
Here are the answers to the worksheet:
- A car engine converts chemical energy from gasoline into mechanical energy. If the engine has an efficiency of 20%, how much mechanical energy is produced from 100 J of chemical energy?
Answer: 20 J
- A solar panel converts sunlight into electrical energy. If the panel has an area of 10 m^2 and receives 500 W/m^2 of sunlight, how much electrical energy is produced in 1 hour?
Answer: 18000 J
- A power plant converts chemical energy from coal into electrical energy. How much electrical energy is produced from 1000 kg of coal?
Answer: 3000000 J
- A car engine converts chemical energy from gasoline into mechanical energy. How much mechanical energy is produced from 100 L of gasoline?
Answer: 100000 J
FAQ Section
What is energy transformation?
+Energy transformation is the process of converting energy from one form to another.
What are the different types of energy?
+There are several types of energy, including kinetic energy, potential energy, thermal energy, electrical energy, chemical energy, and nuclear energy.
What is the first law of thermodynamics?
+The first law of thermodynamics states that energy cannot be created or destroyed, only converted from one form to another.
Related Terms:
- Energy Transformation worksheet Grade 7
- Energy transformations worksheet PDF
- Ipod energy transformation
- The sun energy transformation
- Wind turbine energy transformation