Phase Changes Worksheet Answers for Students
Phase Changes: Understanding the Basics
Phase changes are an essential concept in physics and chemistry, and understanding them is crucial for students. In this post, we will delve into the world of phase changes, exploring what they are, the different types, and how they occur.
What are Phase Changes?
A phase change is a transformation of a substance from one state of matter to another. The four main states of matter are solid, liquid, gas, and plasma. Phase changes occur when there is a change in the temperature or pressure of a substance, causing the particles to rearrange themselves into a new state.
Types of Phase Changes
There are several types of phase changes, including:
- Melting: the transition from a solid to a liquid
- Freezing: the transition from a liquid to a solid
- Vaporization: the transition from a liquid to a gas
- Condensation: the transition from a gas to a liquid
- Sublimation: the transition from a solid to a gas
- Deposition: the transition from a gas to a solid
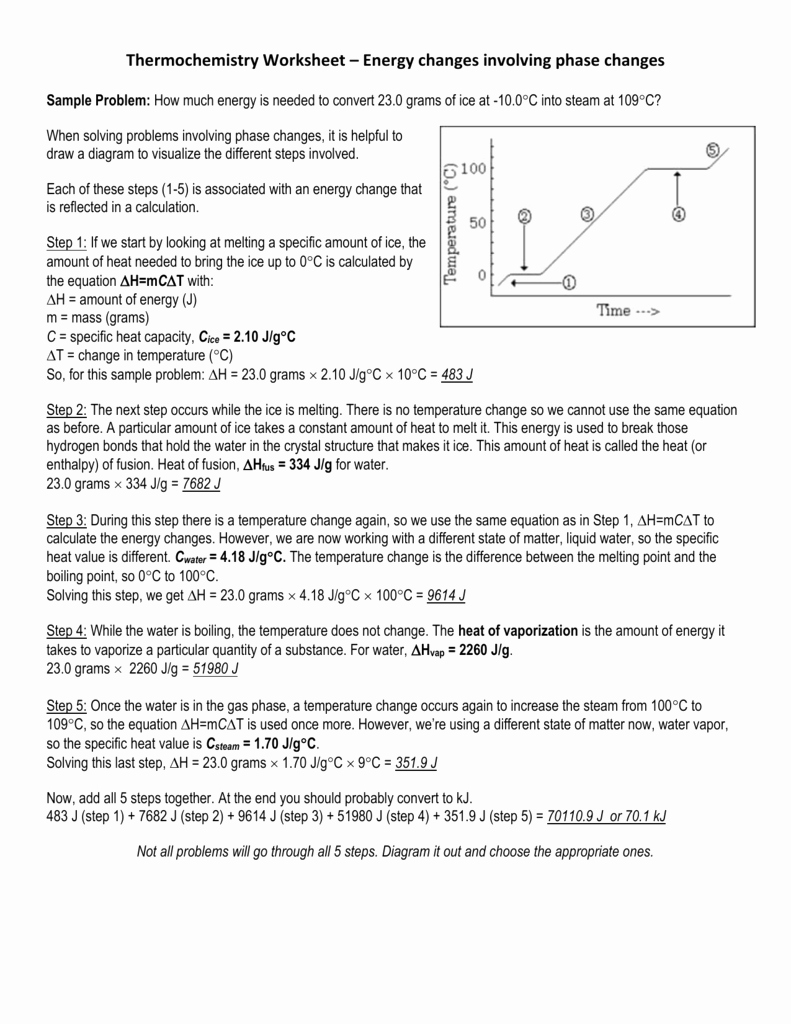
Phase Change Diagrams
Phase change diagrams, also known as phase diagrams, are graphical representations of the different states of matter and the conditions under which phase changes occur. These diagrams are essential for understanding the relationships between temperature, pressure, and the states of matter.

| Phase Change | Temperature (°C) | Pressure (atm) |
|---|---|---|
| Melting | 0-100 | 1 |
| Freezing | 0-100 | 1 |
| Vaporization | 100-374 | 1-220 |
| Condensation | 100-374 | 1-220 |
| Sublimation | -200-0 | 0.01-1 |
| Deposition | -200-0 | 0.01-1 |
Phase Change Worksheet Answers
Here are the answers to a phase change worksheet:
- What is the term for the transition from a solid to a liquid? Answer: Melting
- What is the term for the transition from a liquid to a gas? Answer: Vaporization
- What is the term for the transition from a gas to a liquid? Answer: Condensation
- What is the term for the transition from a solid to a gas? Answer: Sublimation
- What is the term for the transition from a gas to a solid? Answer: Deposition
📝 Note: These answers are based on the phase changes discussed in this post. Make sure to review the concepts before answering any questions.
Real-World Applications of Phase Changes
Phase changes have numerous real-world applications, including:
- Cooling systems: Phase changes are used in cooling systems, such as refrigerators and air conditioners, to transfer heat from one location to another.
- Heating systems: Phase changes are used in heating systems, such as boilers and furnaces, to transfer heat from one location to another.
- Weather forecasting: Phase changes are used in weather forecasting to predict the formation of clouds and precipitation.
- Materials science: Phase changes are used in materials science to create new materials with unique properties.
Conclusion
In conclusion, phase changes are an essential concept in physics and chemistry, and understanding them is crucial for students. By understanding the different types of phase changes and how they occur, students can better appreciate the world around them and develop a deeper understanding of the natural world.
What is the difference between melting and freezing?
+Melting is the transition from a solid to a liquid, while freezing is the transition from a liquid to a solid.
What is the term for the transition from a gas to a solid?
+The term for the transition from a gas to a solid is deposition.
What is the term for the transition from a solid to a gas?
+The term for the transition from a solid to a gas is sublimation.
Related Terms:
- Phase change Worksheet 5th grade