5 Elements Compounds and Mixtures Worksheet Answers

Understanding Elements, Compounds, and Mixtures
Elements, compounds, and mixtures are the building blocks of chemistry, and understanding the differences between them is crucial for any student of chemistry. In this article, we will explore the definitions and examples of elements, compounds, and mixtures, and provide a worksheet with answers to help you practice your knowledge.
Elements
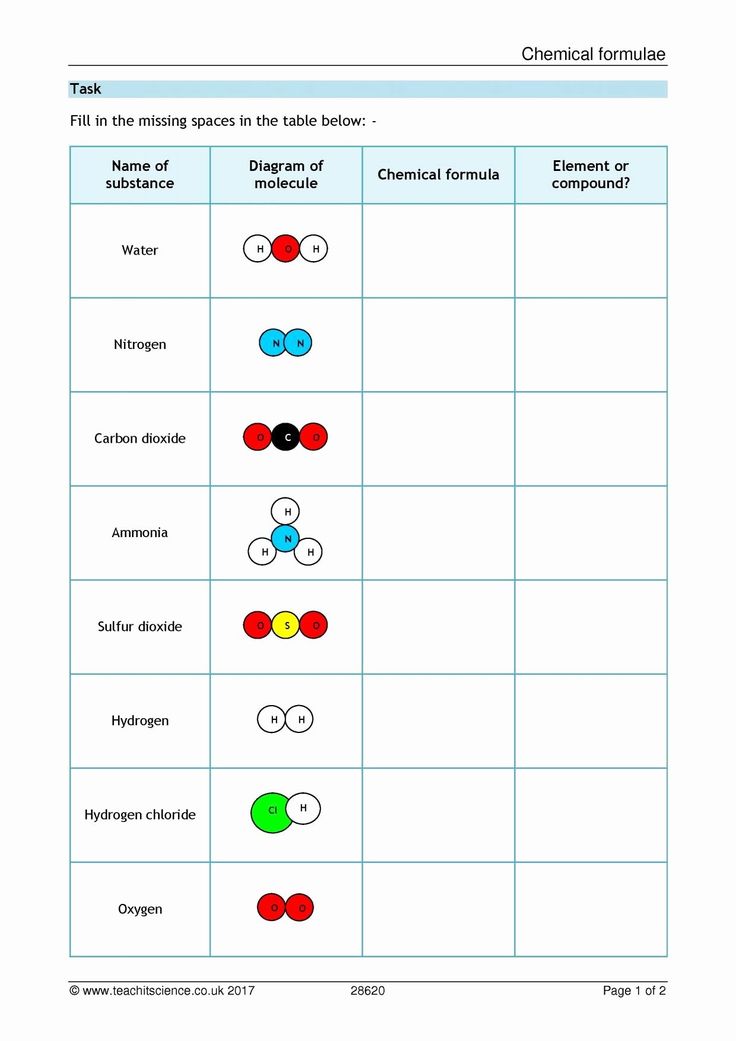
An element is a substance that consists of only one type of atom. It is a pure substance that cannot be broken down into simpler substances by chemical means. Elements are represented by symbols, which are usually one or two letters long. For example, the symbol for oxygen is O, and the symbol for carbon is C.
Compounds
A compound is a substance that is formed when two or more different elements are chemically combined. Compounds have properties that are different from those of their individual elements. For example, water is a compound that is formed when hydrogen and oxygen are combined. The symbol for water is H2O.
Mixtures
A mixture is a physical combination of two or more substances. Mixtures can be separated into their individual components by physical means, such as filtration or distillation. For example, air is a mixture of gases, including nitrogen, oxygen, and carbon dioxide.
Worksheet
Here is a worksheet with 10 questions to help you practice your knowledge of elements, compounds, and mixtures.
| Question | Answer |
|---|---|
| 1. What is the definition of an element? | A substance that consists of only one type of atom. |
| 2. What is the symbol for oxygen? | O |
| 3. What is the definition of a compound? | A substance that is formed when two or more different elements are chemically combined. |
| 4. What is the symbol for water? | H2O |
| 5. What is the definition of a mixture? | A physical combination of two or more substances. |
| 6. What is an example of a mixture? | Air, which is a mixture of gases, including nitrogen, oxygen, and carbon dioxide. |
| 7. What is the difference between a compound and a mixture? | A compound is a substance that is formed when two or more different elements are chemically combined, while a mixture is a physical combination of two or more substances. |
| 8. Can elements be broken down into simpler substances by chemical means? | No, elements are pure substances that cannot be broken down into simpler substances by chemical means. |
| 9. Can compounds be separated into their individual components by physical means? | No, compounds cannot be separated into their individual components by physical means. |
| 10. Can mixtures be separated into their individual components by physical means? | Yes, mixtures can be separated into their individual components by physical means, such as filtration or distillation. |
💡 Note: This worksheet is meant to be a study guide and should not be used as a substitute for actual learning and practice.
In summary, elements are pure substances that consist of only one type of atom, compounds are substances that are formed when two or more different elements are chemically combined, and mixtures are physical combinations of two or more substances. By understanding the differences between these three concepts, you will be well on your way to becoming a master of chemistry.
What is the difference between an element and a compound?
+An element is a substance that consists of only one type of atom, while a compound is a substance that is formed when two or more different elements are chemically combined.
Can mixtures be separated into their individual components?
+Yes, mixtures can be separated into their individual components by physical means, such as filtration or distillation.
What is an example of a compound?
+Water is an example of a compound, which is formed when hydrogen and oxygen are chemically combined.