5 Ways to Master Oxidation Numbers

Understanding Oxidation Numbers
Oxidation numbers are a fundamental concept in chemistry, and mastering them is crucial for any student or professional in the field. Oxidation numbers are a way to keep track of the transfer of electrons during a chemical reaction. They are a measure of the degree of oxidation of an atom in a molecule or ion. In this blog post, we will explore five ways to master oxidation numbers and make them a breeze to work with.
1. Understand the Basics
Before diving into the world of oxidation numbers, it’s essential to understand the basics. Oxidation numbers are assigned to each atom in a molecule or ion, and they can be positive, negative, or zero. The oxidation number of an atom is a measure of its electronegativity, which is its ability to attract electrons.
Here are the basic rules for assigning oxidation numbers:
- Free elements have an oxidation number of 0.
- Monatomic ions have an oxidation number equal to their charge.
- Oxygen has an oxidation number of -2, except in peroxides where it is -1.
- Hydrogen has an oxidation number of +1, except in hydrides where it is -1.
- The sum of the oxidation numbers of all atoms in a molecule or ion must be equal to the charge of the molecule or ion.
📝 Note: Understanding the basics is crucial for mastering oxidation numbers. Make sure you memorize the rules and practice assigning oxidation numbers to simple molecules and ions.
2. Practice Assigning Oxidation Numbers
Practice makes perfect, and assigning oxidation numbers is no exception. Start with simple molecules and ions, and gradually move on to more complex ones. Here are some examples to get you started:
- H2O: Oxygen has an oxidation number of -2, and hydrogen has an oxidation number of +1. The sum of the oxidation numbers is 0, which is the charge of the molecule.
- NaCl: Sodium has an oxidation number of +1, and chlorine has an oxidation number of -1. The sum of the oxidation numbers is 0, which is the charge of the molecule.
- Fe2O3: Iron has an oxidation number of +3, and oxygen has an oxidation number of -2. The sum of the oxidation numbers is 0, which is the charge of the molecule.
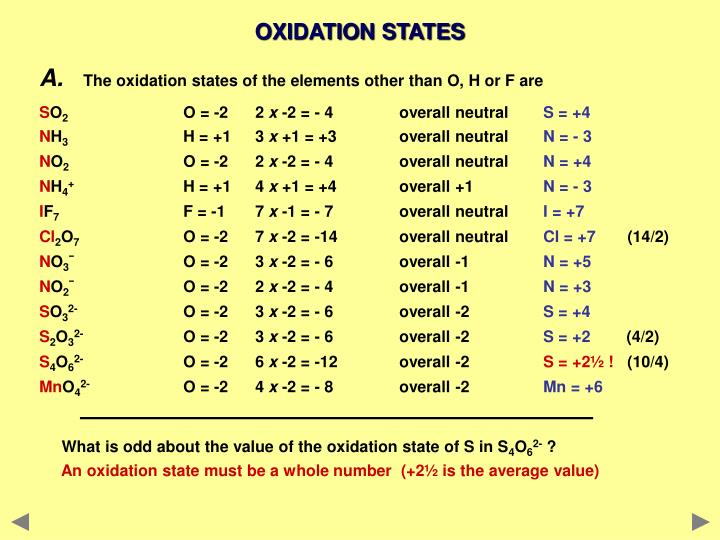
| Molecule/Ion | Oxidation Numbers | Charge |
|---|---|---|
| H2O | O = -2, H = +1 | 0 |
| NaCl | Na = +1, Cl = -1 | 0 |
| Fe2O3 | Fe = +3, O = -2 | 0 |
📝 Note: Practice assigning oxidation numbers to as many molecules and ions as you can. This will help you develop a deeper understanding of the concept and improve your problem-solving skills.
3. Use Oxidation Number Charts
Oxidation number charts are a great way to visualize the relationships between different elements and their oxidation numbers. These charts can be found online or in chemistry textbooks. Here is an example of an oxidation number chart:
| Element | Oxidation Number |
|---|---|
| H | +1, -1 |
| O | -2, -1 |
| Na | +1 |
| Cl | -1 |
| Fe | +2, +3 |
| Cu | +1, +2 |
📝 Note: Oxidation number charts are a great resource for quick reference. Make sure you understand how to read and use them effectively.
4. Learn to Identify Redox Reactions
Redox reactions involve the transfer of electrons between atoms, and oxidation numbers are used to keep track of these electrons. Here are some tips for identifying redox reactions:
- Look for the transfer of electrons between atoms.
- Check if the oxidation numbers of the atoms change during the reaction.
- Identify the oxidizing and reducing agents.
Example:
2Na + Cl2 → 2NaCl
In this reaction, sodium is oxidized (loses electrons), and chlorine is reduced (gains electrons).
📝 Note: Learning to identify redox reactions is crucial for mastering oxidation numbers. Practice identifying redox reactions and determining the oxidation numbers of the atoms involved.
5. Apply Oxidation Numbers to Real-World Scenarios
Oxidation numbers have numerous applications in real-world scenarios, such as:
- Chemistry of the environment: Understanding the oxidation numbers of elements in the environment helps us understand the chemistry of pollution and climate change.
- Biochemistry: Oxidation numbers play a crucial role in understanding the chemistry of living organisms.
- Materials science: Understanding the oxidation numbers of elements helps us develop new materials with specific properties.
Example:
The rusting of iron is a redox reaction that involves the transfer of electrons between iron and oxygen.
4Fe + 3O2 → 2Fe2O3
In this reaction, iron is oxidized (loses electrons), and oxygen is reduced (gains electrons).
📝 Note: Applying oxidation numbers to real-world scenarios helps you develop a deeper understanding of the concept and its relevance to the world around us.
By following these five tips, you’ll be well on your way to mastering oxidation numbers. Remember to practice assigning oxidation numbers, use oxidation number charts, learn to identify redox reactions, and apply oxidation numbers to real-world scenarios.
And that’s a wrap! With these tips, you’ll be able to tackle even the toughest oxidation number problems with ease.
What are oxidation numbers?
+Oxidation numbers are a measure of the degree of oxidation of an atom in a molecule or ion. They are a way to keep track of the transfer of electrons during a chemical reaction.
How do I assign oxidation numbers?
+To assign oxidation numbers, follow the basic rules: free elements have an oxidation number of 0, monatomic ions have an oxidation number equal to their charge, and oxygen has an oxidation number of -2, except in peroxides where it is -1.
What are some common applications of oxidation numbers?
+Oxidation numbers have numerous applications in real-world scenarios, such as chemistry of the environment, biochemistry, and materials science.