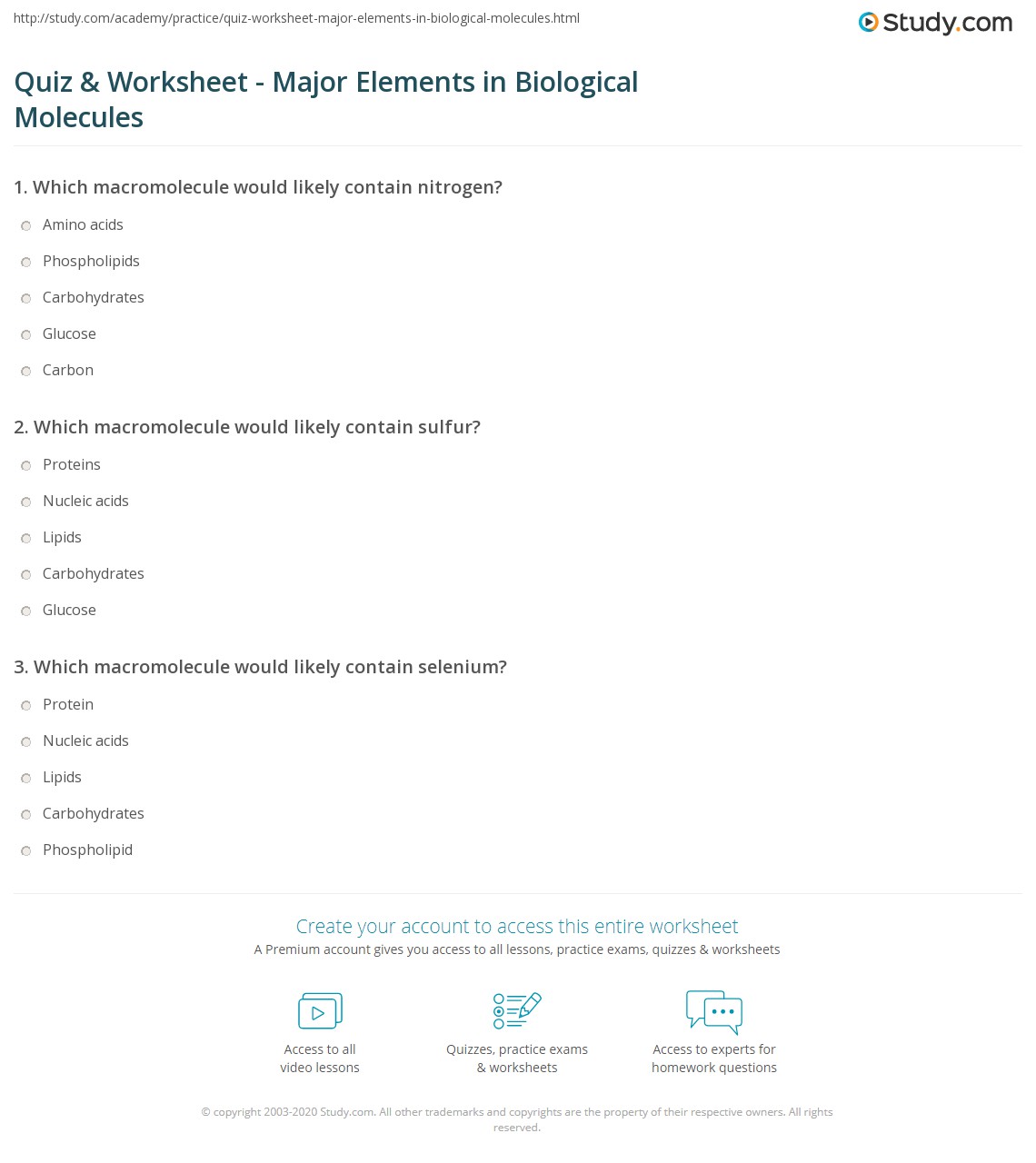
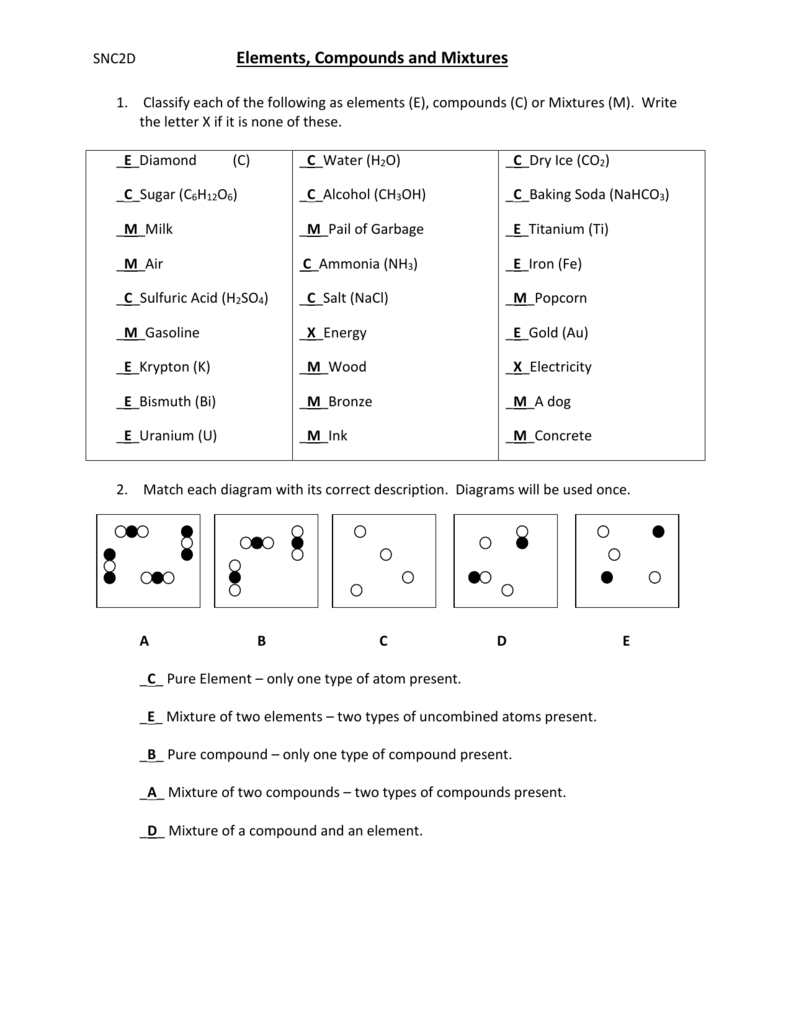
Element Compounds and Mixtures Worksheet Answer Key

Understanding Element Compounds and Mixtures
Elements are the simplest substances in chemistry, and they can combine to form compounds or mixtures. In this worksheet, we will explore the difference between element compounds and mixtures, and provide answers to common questions.
What are Element Compounds?
Element compounds are substances formed when two or more different elements are chemically bonded together. This means that the elements share electrons to form a strong chemical bond. Element compounds have properties that are different from those of the individual elements.
For example, when sodium (Na) and chlorine (Cl) combine, they form sodium chloride (NaCl), also known as table salt.
Characteristics of Element Compounds:
- Formed by chemical bonds between elements
- Have properties different from individual elements
- Can be broken down into individual elements through chemical reactions
What are Mixtures?
Mixtures are physical combinations of two or more substances that are not chemically bonded together. The components of a mixture retain their individual properties and can be separated through physical means, such as filtration or distillation.
For example, a mixture of sand and water can be separated by filtration, and the sand and water will retain their individual properties.
Characteristics of Mixtures:
- Physical combination of substances
- Components retain individual properties
- Can be separated through physical means
Key Differences between Element Compounds and Mixtures
| Element Compounds | Mixtures | |
|---|---|---|
| Formation | Chemical bonds between elements | Physical combination of substances |
| Properties | Different from individual elements | Components retain individual properties |
| Separation | Broken down through chemical reactions | Separated through physical means |
👍 Note: Understanding the difference between element compounds and mixtures is crucial in chemistry, as it helps us identify and classify substances accurately.
Examples of Element Compounds and Mixtures
- Element compounds:
- Water (H2O)
- Carbon dioxide (CO2)
- Ammonia (NH3)
- Mixtures:
- Air (a mixture of gases)
- Soil (a mixture of minerals and organic matter)
- Seawater (a mixture of water and salts)
Conclusion
In conclusion, element compounds and mixtures are two distinct types of substances in chemistry. Element compounds are formed through chemical bonds between elements, while mixtures are physical combinations of substances. Understanding the characteristics and differences between these two types of substances is essential for identifying and classifying substances accurately.
What is the main difference between element compounds and mixtures?
+The main difference between element compounds and mixtures is the type of bond between the components. Element compounds are formed through chemical bonds, while mixtures are physical combinations of substances.
Can element compounds be broken down into individual elements?
+Yes, element compounds can be broken down into individual elements through chemical reactions.
What is an example of a mixture?
+An example of a mixture is air, which is a physical combination of gases such as nitrogen, oxygen, and carbon dioxide.
Related Terms:
- Element and compound
- Element compound and mixture
- Quiz element compound mixture
- Homogeneous and heterogeneous mixtures worksheet