5 Easy Steps to Master Electron Configuration
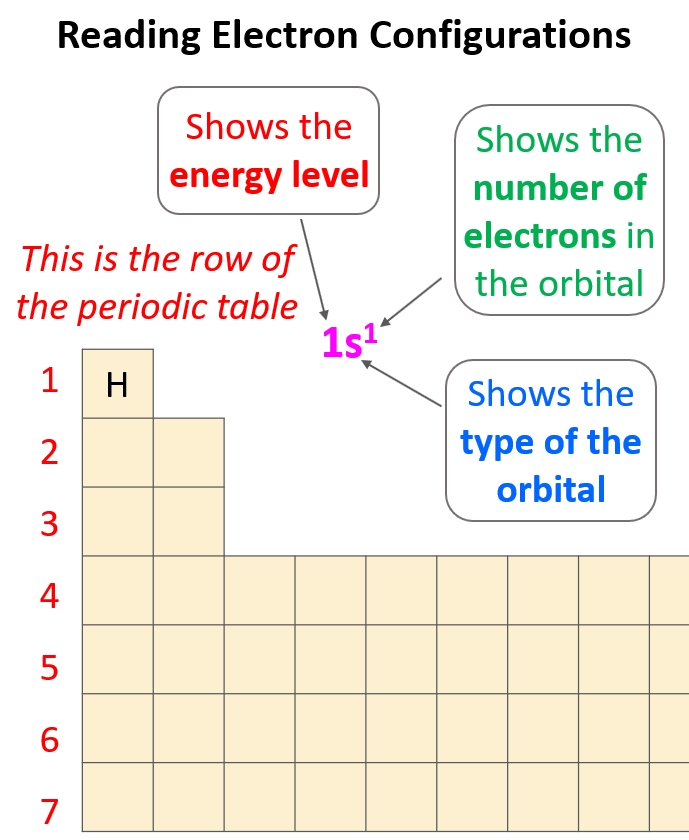
Understanding Electron Configuration: A Fundamental Concept in Chemistry
Electron configuration is a crucial concept in chemistry that describes the distribution of electrons in an atom. It is a fundamental idea that helps us understand the behavior of atoms and molecules, and it is essential for understanding various chemical properties and reactions. Mastering electron configuration can seem daunting, but with practice and the right approach, it can be made easy. In this article, we will break down the process of electron configuration into five easy steps.
Step 1: Determine the Number of Electrons
The first step in electron configuration is to determine the number of electrons in an atom. This can be done by looking at the atomic number of the element, which is the number of protons in the nucleus of an atom. The number of electrons in a neutral atom is equal to the number of protons, so if you know the atomic number, you can determine the number of electrons.
For example, if we are working with the element sodium (Na), which has an atomic number of 11, we know that it has 11 electrons.
Important Electrons to Consider
When determining the number of electrons, it is essential to consider the following:
- Valence electrons: These are the electrons in the outermost energy level of an atom. They play a crucial role in chemical bonding and reactions.
- Core electrons: These are the electrons in the inner energy levels of an atom. They are not typically involved in chemical bonding and reactions.
🔍 Note: The number of electrons in an atom can also be determined by looking at the periodic table. The number of electrons in an atom is equal to the number of protons, which is indicated by the atomic number on the periodic table.
Step 2: Identify the Energy Levels
The second step in electron configuration is to identify the energy levels or electron shells in an atom. Energy levels are the regions around an atom where electrons are found. Each energy level has a specific capacity, and electrons fill the lowest available energy levels first.
The main energy levels in an atom are:
- 1s: The first energy level, which can hold up to 2 electrons.
- 2s: The second energy level, which can hold up to 2 electrons.
- 2p: The second energy level, which can hold up to 6 electrons.
- 3s: The third energy level, which can hold up to 2 electrons.
- 3p: The third energy level, which can hold up to 6 electrons.
Energy Level Capacities
Here is a summary of the energy level capacities:
| Energy Level | Capacity |
|---|---|
| 1s | 2 |
| 2s | 2 |
| 2p | 6 |
| 3s | 2 |
| 3p | 6 |
Step 3: Apply the Aufbau Principle
The third step in electron configuration is to apply the Aufbau principle, which states that electrons fill the lowest available energy levels first. This means that electrons will occupy the 1s energy level before moving to the 2s energy level, and so on.
For example, if we are working with the element sodium (Na), which has 11 electrons, we can apply the Aufbau principle as follows:
- 2 electrons in the 1s energy level
- 2 electrons in the 2s energy level
- 6 electrons in the 2p energy level
- 1 electron in the 3s energy level
Step 4: Apply the Pauli Exclusion Principle
The fourth step in electron configuration is to apply the Pauli exclusion principle, which states that no two electrons in an atom can have the same set of quantum numbers. Quantum numbers describe the energy, shape, and orientation of an electron’s orbital.
The Pauli exclusion principle means that each orbital can hold a maximum of two electrons, which must have opposite spins.
For example, if we are working with the element sodium (Na), which has 11 electrons, we can apply the Pauli exclusion principle as follows:
- 2 electrons in the 1s energy level, with opposite spins
- 2 electrons in the 2s energy level, with opposite spins
- 6 electrons in the 2p energy level, with opposite spins
- 1 electron in the 3s energy level, with one spin
Step 5: Write the Electron Configuration
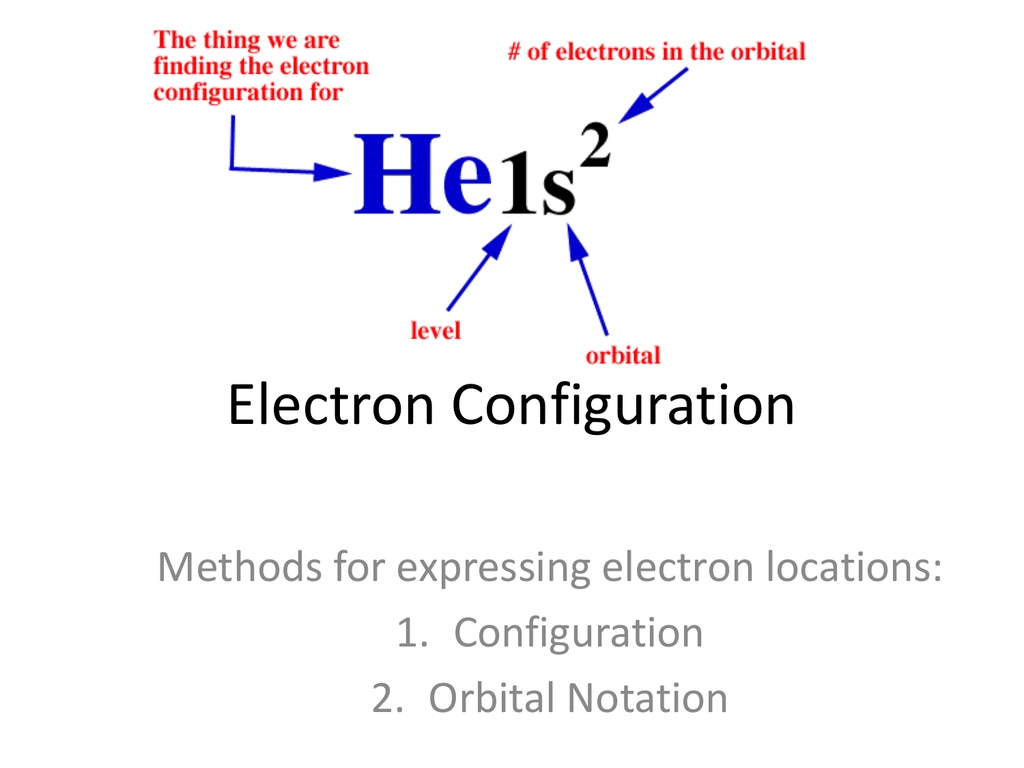
The final step in electron configuration is to write the electron configuration notation. This notation describes the distribution of electrons in an atom.
For example, if we are working with the element sodium (Na), which has 11 electrons, we can write the electron configuration notation as follows:
1s² 2s² 2p⁶ 3s¹
This notation indicates that sodium has:
- 2 electrons in the 1s energy level
- 2 electrons in the 2s energy level
- 6 electrons in the 2p energy level
- 1 electron in the 3s energy level
🔍 Note: The electron configuration notation is a shorthand way of describing the distribution of electrons in an atom. It is essential to understand the notation to be able to write the electron configuration correctly.
In conclusion, mastering electron configuration requires practice and a step-by-step approach. By following the five easy steps outlined in this article, you can become proficient in writing electron configurations and understanding the behavior of atoms and molecules.
What is the Aufbau principle?
+The Aufbau principle states that electrons fill the lowest available energy levels first. This means that electrons will occupy the 1s energy level before moving to the 2s energy level, and so on.
What is the Pauli exclusion principle?
+The Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers. Quantum numbers describe the energy, shape, and orientation of an electron’s orbital.
How do I write the electron configuration notation?
+The electron configuration notation is a shorthand way of describing the distribution of electrons in an atom. It is written in the format of [energy level] [number of electrons]. For example, 1s² 2s² 2p⁶ 3s¹.