Egg In Vinegar Experiment Worksheet

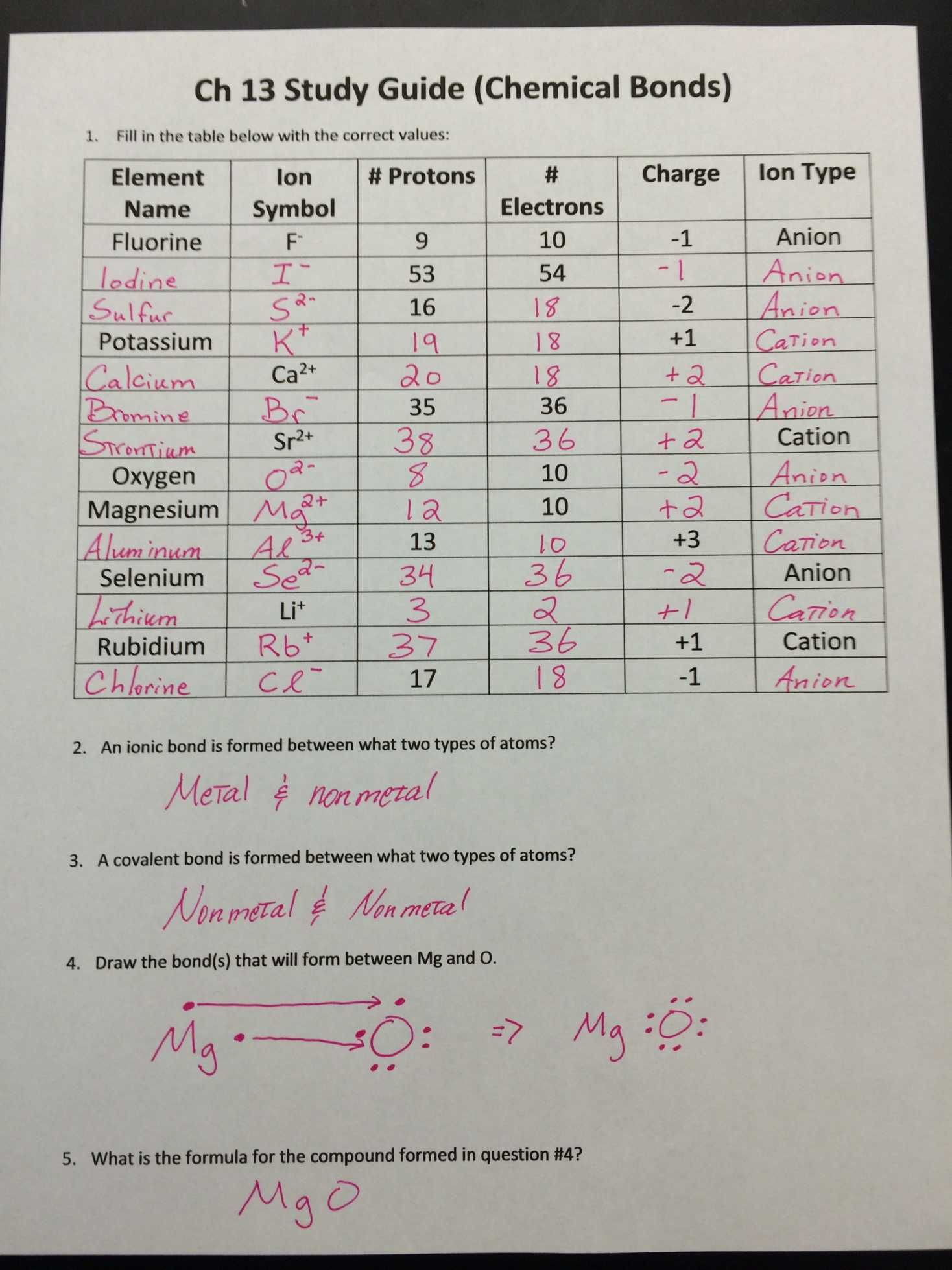
Introduction to the Egg in Vinegar Experiment
The egg in vinegar experiment is a classic science demonstration that showcases the power of acid on calcium carbonate-based structures. In this experiment, we will be exploring how vinegar affects the shell of an egg, leading to some fascinating observations and conclusions.
Materials Needed
- 1 hard-boiled egg
- 1 cup of vinegar (white or apple cider)
- 1 cup or a container with a lid
- A spoon or tongs for handling the egg
- Optional: food coloring, a timer, and a measuring tape
Step-by-Step Instructions
- Prepare the Egg: Start by carefully placing the hard-boiled egg into the cup or container.
- Add Vinegar: Pour the cup of vinegar over the egg, making sure that the egg is completely submerged.
- Observe and Wait: Place the lid on the container and let it sit for 2-3 days. You can observe the egg after 24 hours, but the best results will be seen after 48-72 hours.
- Record Your Observations: Take note of any changes in the eggshell, such as cracks, softening, or dissolution. You can also measure the egg’s circumference and length before and after the experiment using a measuring tape.
- Optional: Add Food Coloring: If you’d like to add an extra layer of excitement to the experiment, you can add a few drops of food coloring to the vinegar before pouring it over the egg. This will help to visualize the acid’s effect on the eggshell.
What's Happening in the Experiment
The eggshell is primarily composed of calcium carbonate, which is a weak base. Vinegar, on the other hand, is a weak acid. When the acid comes into contact with the base, a chemical reaction occurs, resulting in the breakdown of the calcium carbonate.
Chemical Reaction:
CaCO3 (calcium carbonate) + CH3COOH (acetic acid) → Ca(CH3COO)2 (calcium acetate) + H2O (water) + CO2 (carbon dioxide)
As the acid breaks down the calcium carbonate, the eggshell begins to dissolve, leading to the observed changes.
Notes
📝 Note: Make sure to handle the egg gently when placing it in the container, as rough handling can cause the egg to crack.
💡 Note: If you're using a clear container, you can observe the egg's transformation more easily. However, be aware that light can affect the experiment's outcome.
Results and Discussion
After 2-3 days, you should observe significant changes in the eggshell, including:
- Softening or dissolution of the eggshell
- Cracks or breaks in the shell
- A decrease in the egg’s circumference and length
These results demonstrate the power of acid on calcium carbonate-based structures. The egg in vinegar experiment is an excellent way to illustrate chemical reactions and the importance of pH in everyday life.
Conclusion
The egg in vinegar experiment is a fun and educational way to explore the world of chemistry. By following these simple steps and observing the changes in the eggshell, you can gain a deeper understanding of acid-base reactions and their effects on different materials. Remember to record your observations and discuss the results with your peers to reinforce your learning.
What is the purpose of the egg in vinegar experiment?
+The egg in vinegar experiment demonstrates the effect of acid on calcium carbonate-based structures, illustrating chemical reactions and the importance of pH in everyday life.
Why does the eggshell dissolve in vinegar?
+The eggshell is composed of calcium carbonate, which is a weak base. Vinegar is a weak acid. When the acid comes into contact with the base, a chemical reaction occurs, resulting in the breakdown of the calcium carbonate.
What can I use instead of vinegar in the experiment?
+While vinegar is the most common choice, you can also use other acidic substances like lemon juice or soda. However, keep in mind that the results may vary depending on the acidity level and type of acid used.