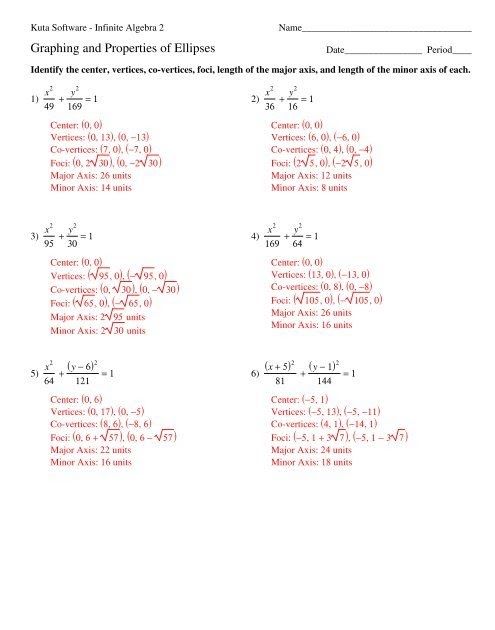
5 Essential Equations for Gas Law Mastery
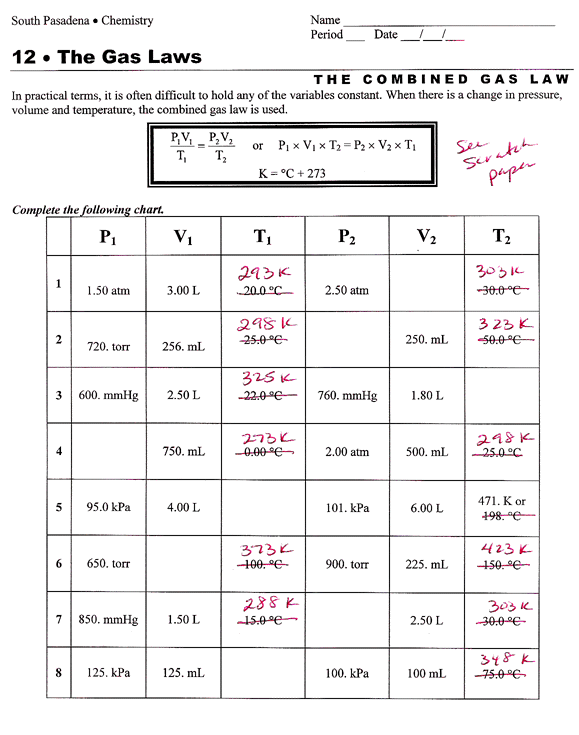
Unlocking the Secrets of Gas Behavior: Essential Equations for Mastery
Gas laws are fundamental principles in chemistry and physics that describe the behavior of gases. Mastering these laws is crucial for understanding various natural phenomena and industrial processes. In this article, we will explore five essential equations for gas law mastery, which will help you grasp the intricacies of gas behavior and solve complex problems with ease.
1. Boyle's Law: The Relationship Between Pressure and Volume
Boyle’s Law states that, at constant temperature, the volume of a gas is inversely proportional to the pressure. Mathematically, this is expressed as:
P1V1 = P2V2
Where:
- P1 and P2 are the initial and final pressures
- V1 and V2 are the initial and final volumes
This equation shows that as the pressure of a gas increases, its volume decreases, and vice versa. Boyle’s Law is a fundamental concept in understanding gas behavior and is widely used in various industrial applications.
2. Charles' Law: The Relationship Between Temperature and Volume
Charles’ Law states that, at constant pressure, the volume of a gas is directly proportional to the temperature. Mathematically, this is expressed as:
V1 / T1 = V2 / T2
Where:
- V1 and V2 are the initial and final volumes
- T1 and T2 are the initial and final temperatures (in Kelvin)
This equation shows that as the temperature of a gas increases, its volume also increases, and vice versa. Charles’ Law is essential in understanding the behavior of gases in various temperature ranges.
3. Gay-Lussac's Law: The Relationship Between Pressure and Temperature
Gay-Lussac’s Law states that, at constant volume, the pressure of a gas is directly proportional to the temperature. Mathematically, this is expressed as:
P1 / T1 = P2 / T2
Where:
- P1 and P2 are the initial and final pressures
- T1 and T2 are the initial and final temperatures (in Kelvin)
This equation shows that as the temperature of a gas increases, its pressure also increases, and vice versa. Gay-Lussac’s Law is crucial in understanding the behavior of gases in various industrial processes.
4. Avogadro's Law: The Relationship Between Volume and Number of Moles
Avogadro’s Law states that, at constant pressure and temperature, the volume of a gas is directly proportional to the number of moles. Mathematically, this is expressed as:
V1 / n1 = V2 / n2
Where:
- V1 and V2 are the initial and final volumes
- n1 and n2 are the initial and final number of moles
This equation shows that as the number of moles of a gas increases, its volume also increases, and vice versa. Avogadro’s Law is essential in understanding the behavior of gases in various chemical reactions.
5. The Ideal Gas Law: A Comprehensive Equation
The Ideal Gas Law is a comprehensive equation that combines the principles of Boyle’s Law, Charles’ Law, and Avogadro’s Law. Mathematically, this is expressed as:
PV = nRT
Where:
- P is the pressure
- V is the volume
- n is the number of moles
- R is the gas constant
- T is the temperature (in Kelvin)
This equation shows that the behavior of a gas can be predicted by understanding the relationships between pressure, volume, number of moles, and temperature. The Ideal Gas Law is widely used in various industrial applications and is a fundamental concept in understanding gas behavior.
💡 Note: These equations are essential in understanding gas behavior and are widely used in various industrial applications. Mastering these equations will help you solve complex problems with ease and gain a deeper understanding of gas behavior.
In conclusion, mastering the five essential equations for gas law mastery is crucial for understanding the behavior of gases. By grasping these equations, you will be able to solve complex problems with ease and gain a deeper understanding of gas behavior. Remember to practice solving problems using these equations to reinforce your understanding.
What is the difference between Boyle’s Law and Charles’ Law?
+Boyle’s Law describes the relationship between pressure and volume, while Charles’ Law describes the relationship between temperature and volume.
What is the Ideal Gas Law?
+The Ideal Gas Law is a comprehensive equation that combines the principles of Boyle’s Law, Charles’ Law, and Avogadro’s Law. It describes the behavior of a gas in terms of pressure, volume, number of moles, and temperature.
Why is it essential to master the gas laws?
+Mastering the gas laws is essential in understanding various natural phenomena and industrial processes. It helps you solve complex problems with ease and gain a deeper understanding of gas behavior.
Related Terms:
- Solubility Chart Worksheet
- Solubility Worksheet PDF
- Solubility Worksheet middle school
- Solutions Worksheet