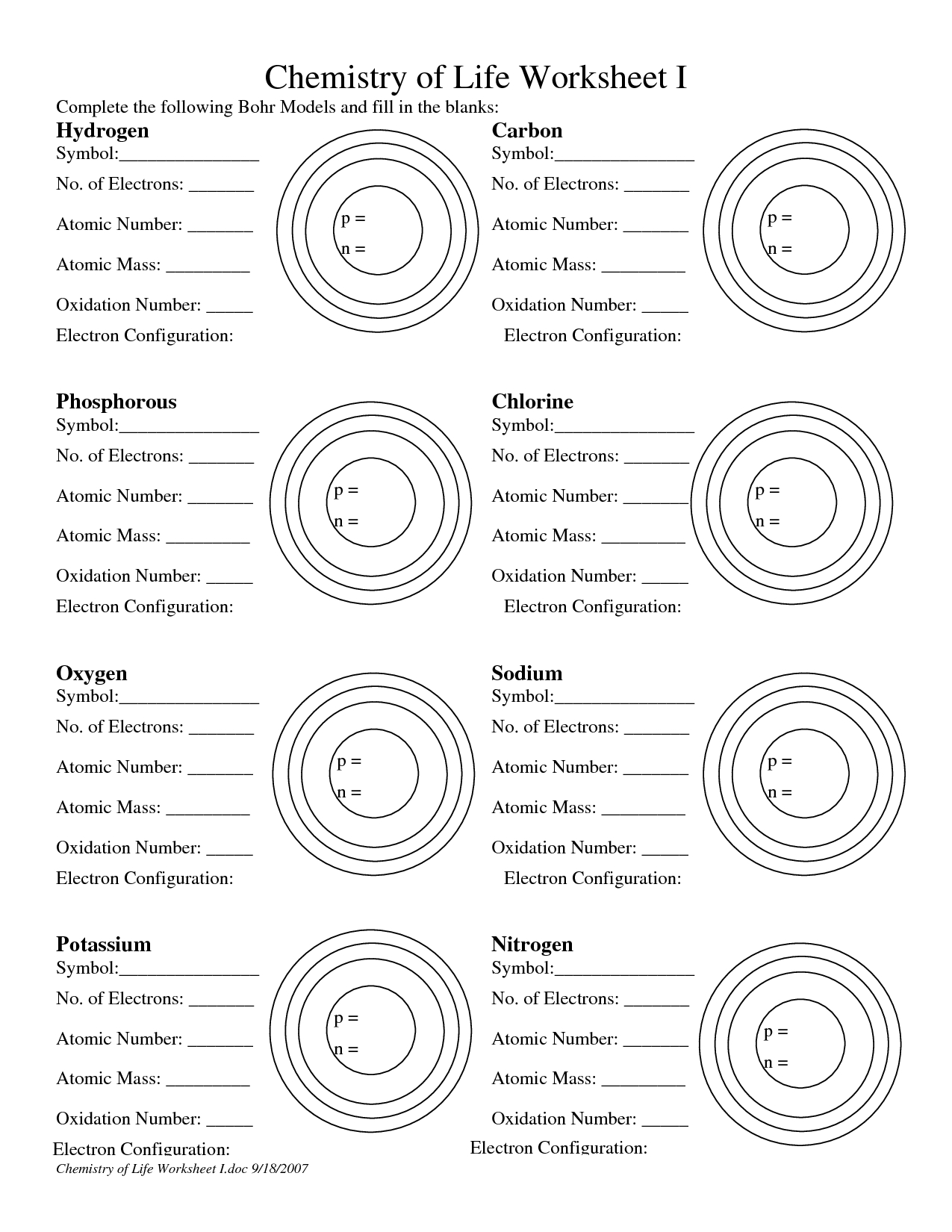
Bohr Model Worksheet: Understanding Atomic Structure Made Easy

Introduction to the Bohr Model
The Bohr model, developed by Niels Bohr in 1913, is a simplified representation of the atomic structure. This model helps us understand the arrangement of electrons in an atom and how they interact with the nucleus. The Bohr model is an essential concept in chemistry and physics, and it’s crucial to grasp the basics to build a strong foundation in these subjects.
Understanding the Bohr Model
The Bohr model consists of three main components:
- Nucleus: The nucleus is the central part of the atom, containing protons and neutrons. Protons have a positive charge, while neutrons are neutral.
- Electrons: Electrons are negatively charged particles that orbit the nucleus in energy levels or shells.
- Energy Levels: Energy levels, also known as electron shells, are the regions around the nucleus where electrons are found. Each energy level has a specific capacity, and electrons in each level have a particular amount of energy.
Key Principles of the Bohr Model
- Energy Quantization: Electrons can only occupy specific energy levels, and they cannot exist between these levels.
- Electron Jumps: Electrons can jump from one energy level to another by absorbing or emitting energy.
- Electron Configuration: The arrangement of electrons in an atom is determined by the energy levels they occupy.
Bohr Model Diagram
Here’s a simplified diagram of the Bohr model:
| Nucleus | Energy Level 1 (n=1) | Energy Level 2 (n=2) | Energy Level 3 (n=3) |
|---|---|---|---|
| Protons and Neutrons | 2 electrons (max) | 8 electrons (max) | 18 electrons (max) |
Notes
💡 Note: The numbers in the diagram represent the maximum number of electrons each energy level can hold.
Energy Levels and Electron Capacity
Each energy level has a specific capacity, which determines the maximum number of electrons it can hold. The first energy level (n=1) can hold up to 2 electrons, the second energy level (n=2) can hold up to 8 electrons, and so on.
Energy Level Capacities
- Energy Level 1 (n=1): 2 electrons
- Energy Level 2 (n=2): 8 electrons
- Energy Level 3 (n=3): 18 electrons
- Energy Level 4 (n=4): 32 electrons
Electron Configuration
To determine the electron configuration of an atom, we need to know the number of electrons in each energy level. The electron configuration is written in a specific notation, where the number of electrons in each energy level is indicated.
Example: Electron Configuration of Hydrogen
Hydrogen has one electron in the first energy level (n=1). The electron configuration is written as 1s¹, where:
- 1 represents the energy level (n=1)
- s represents the type of orbital (s-orbital)
- ¹ represents the number of electrons in that orbital
Conclusion
The Bohr model is a fundamental concept in understanding the atomic structure. By grasping the key principles and components of the Bohr model, we can better comprehend the arrangement of electrons in an atom and how they interact with the nucleus. Remember, the Bohr model is a simplified representation, and there are more advanced models that provide a more accurate description of the atomic structure.
What is the main advantage of the Bohr model?
+The main advantage of the Bohr model is that it provides a simple and intuitive way to understand the atomic structure, making it easier to visualize and predict the behavior of electrons.
What is the difference between the Bohr model and the Rutherford model?
+The Rutherford model, developed by Ernest Rutherford, is an earlier model of the atomic structure that describes the nucleus as a small, dense region at the center of the atom. The Bohr model builds upon the Rutherford model by introducing energy levels and electron jumps.
How does the Bohr model relate to the periodic table?
+The Bohr model helps explain the periodic trends and patterns observed in the periodic table. The energy levels and electron configurations of atoms determine their chemical properties, which are reflected in the periodic table.
Related Terms:
- Momentum sudut
- Bilangan kuantum utama
- Konstanta Planck
- Pi
- Bohr Model Worksheet PDF
- Bohr model Worksheet with answers