5 Key Features of the Bohr Model Explained
The Bohr Model: A Simplified Explanation of Atomic Structure
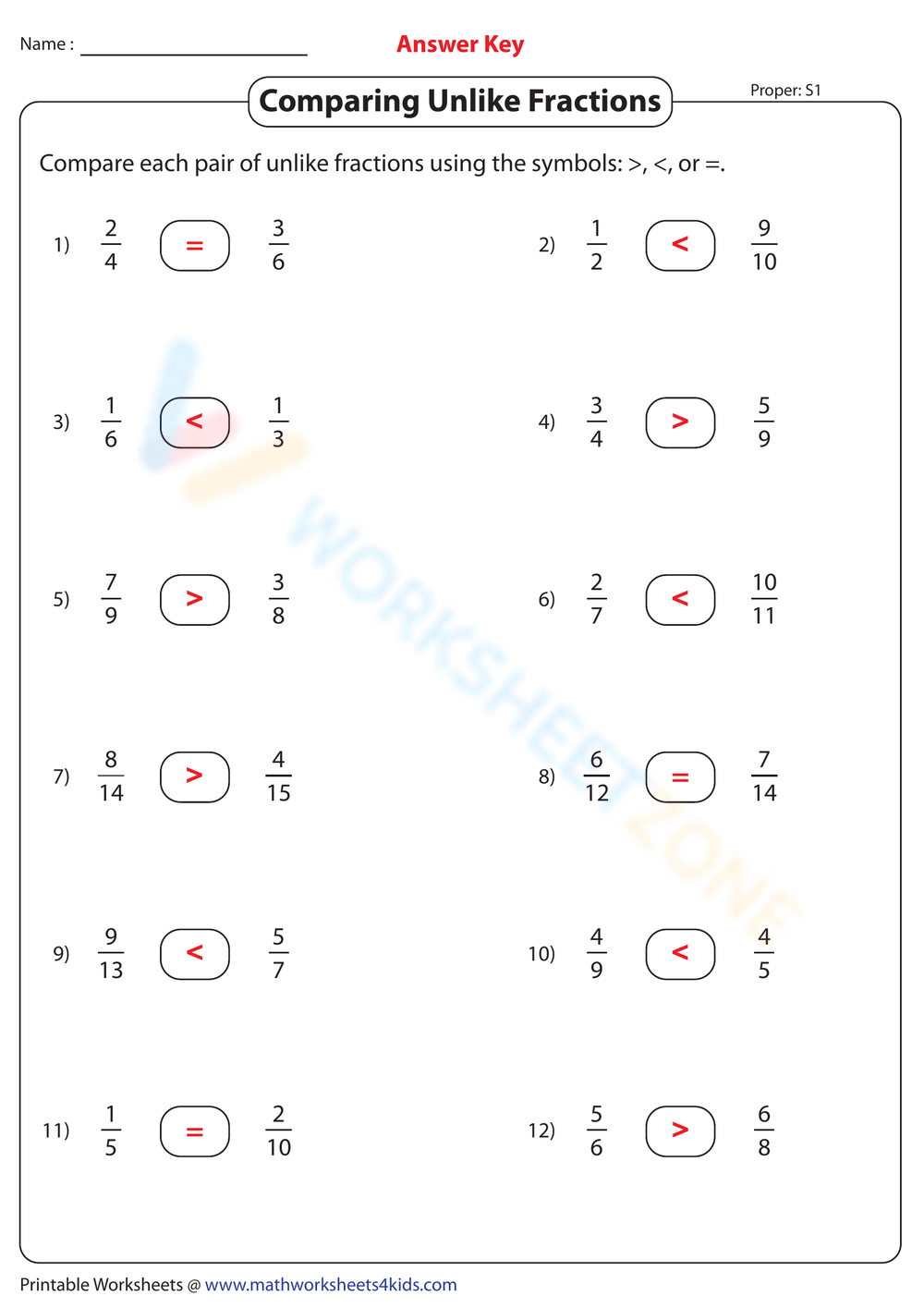
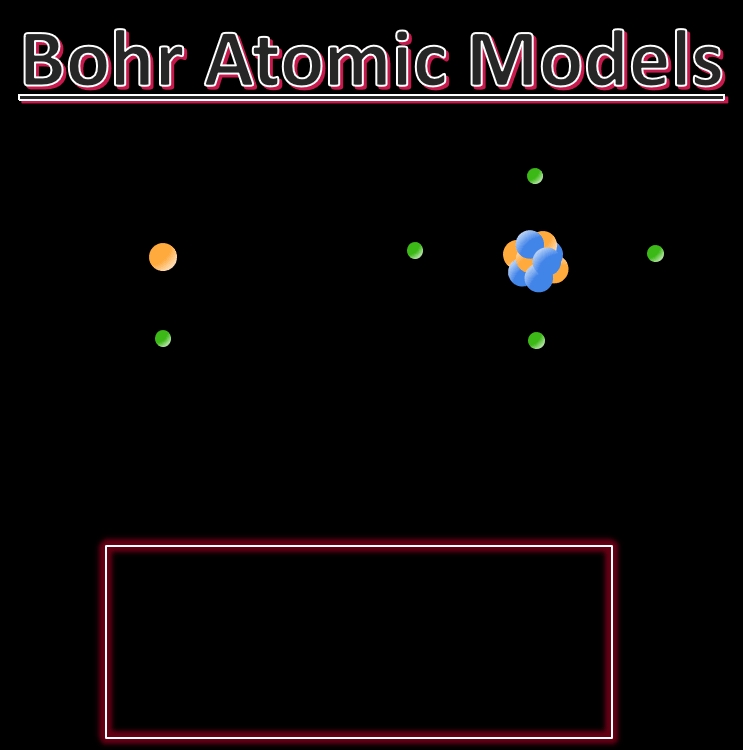
The Bohr model, proposed by Niels Bohr in 1913, is a simplified explanation of the atomic structure. It revolutionized the understanding of atomic physics and paved the way for the development of quantum mechanics. The model describes the atom as a small, heavy nucleus surrounded by electrons in circular orbits. Here are 5 key features of the Bohr model explained in detail:
1. Electron Orbits and Energy Levels
The Bohr model postulates that electrons occupy specific energy levels, or shells, around the nucleus. These energy levels are discrete and correspond to specific orbits. The electrons in each orbit have a specific energy, and they can jump from one orbit to another by emitting or absorbing energy. The energy levels are labeled as n = 1, 2, 3,…, where n is the principal quantum number.
🔹 Note: The Bohr model assumes that electrons occupy fixed orbits, which is not the case in reality. In reality, electrons exist in a cloud of probability, and their positions are uncertain.
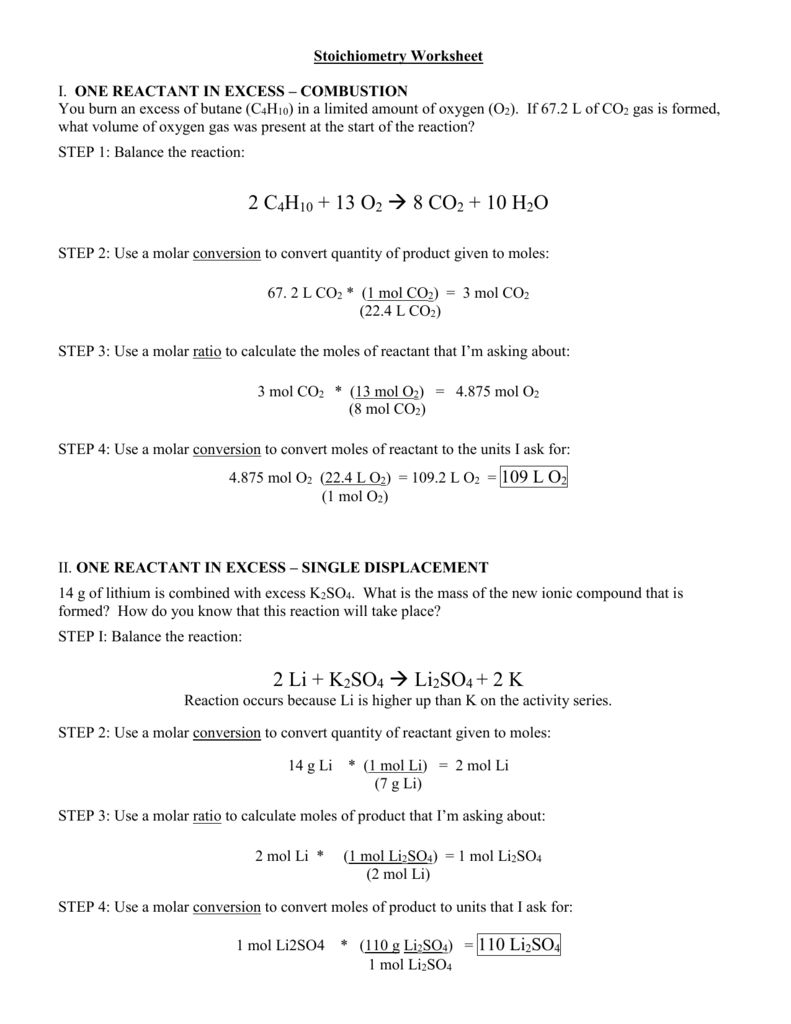
2. Nucleus and Protons
The Bohr model describes the nucleus as a small, heavy, positively charged region at the center of the atom. The nucleus contains protons, which are positively charged particles. The number of protons in the nucleus determines the atomic number of an element, which is unique to each element.
3. Electron Jumps and Spectral Lines
When an electron jumps from one orbit to another, it emits or absorbs energy. This energy is released or absorbed in the form of light, which corresponds to specific spectral lines. The Bohr model explains the origin of spectral lines in the hydrogen spectrum, which was a major achievement at the time.
4. Atomic Radius and Orbit Size
The Bohr model assumes that the atomic radius is determined by the size of the electron orbits. The radius of each orbit is proportional to the square of the principal quantum number (n). The size of the orbits decreases as the energy level decreases.
5. Electron Spin and Magnetic Moment
The Bohr model introduces the concept of electron spin, which is a fundamental property of electrons. Electron spin is a measure of the intrinsic angular momentum of an electron. The magnetic moment of an electron is related to its spin and is a key feature of the Bohr model.
🔹 Note: The Bohr model does not explain the Zeeman effect, which is the splitting of spectral lines in the presence of a magnetic field. This limitation was later addressed by the development of quantum mechanics.
Limitations of the Bohr Model
While the Bohr model was a significant improvement over earlier atomic models, it has several limitations. It does not explain the fine structure of spectral lines, the Zeeman effect, or the anomalous Zeeman effect. Additionally, the model assumes that electrons occupy fixed orbits, which is not the case in reality.
Conclusion
The Bohr model is a simplified explanation of the atomic structure that revolutionized the understanding of atomic physics. While it has several limitations, it provides a foundation for understanding more advanced atomic models, such as quantum mechanics. The 5 key features of the Bohr model explained above provide a comprehensive overview of this fundamental concept in physics.
What is the main assumption of the Bohr model?
+The main assumption of the Bohr model is that electrons occupy fixed orbits around the nucleus.
What is the significance of the Bohr model?
+The Bohr model was a significant improvement over earlier atomic models and provided a foundation for understanding more advanced atomic models, such as quantum mechanics.
What are the limitations of the Bohr model?
+The Bohr model does not explain the fine structure of spectral lines, the Zeeman effect, or the anomalous Zeeman effect. Additionally, the model assumes that electrons occupy fixed orbits, which is not the case in reality.