Bohr Model Diagram Worksheet Answers Key and Solutions
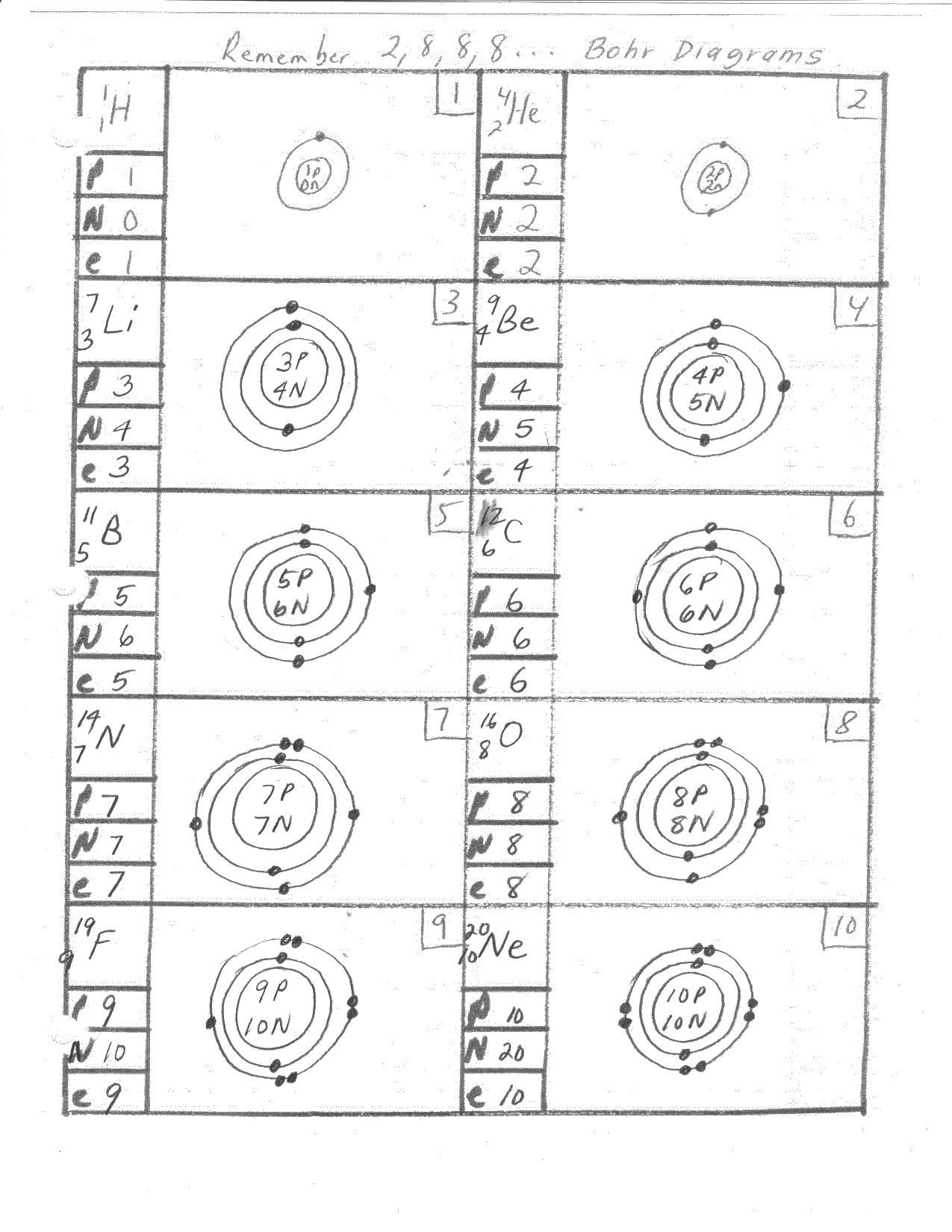
Understanding the Bohr Model Diagram
The Bohr model, proposed by Niels Bohr in 1913, is a simplified model of an atom that depicts the arrangement of electrons in energy levels or shells around the nucleus. This model is essential in understanding the structure of atoms and how they interact with each other. In this article, we will delve into the details of the Bohr model diagram, its components, and provide answers to common worksheet questions.
Components of the Bohr Model Diagram
The Bohr model diagram consists of the following key components:
- Nucleus: The central part of the atom that contains protons and neutrons.
- Energy Levels or Shells: The regions around the nucleus where electrons are found.
- Electrons: Negatively charged particles that occupy specific energy levels.
- Protons: Positively charged particles found in the nucleus.
- Neutrons: Particles with no charge found in the nucleus.
How to Draw a Bohr Model Diagram
Drawing a Bohr model diagram involves the following steps:
- Start with the Nucleus: Draw a small circle to represent the nucleus, which contains protons and neutrons.
- Add Energy Levels: Draw concentric circles around the nucleus to represent the energy levels or shells.
- Determine the Number of Energy Levels: The number of energy levels depends on the period of the element in the periodic table.
- Add Electrons: Place electrons in the energy levels, starting from the innermost level. Each level has a specific capacity:
- First level (1s): 2 electrons
- Second level (2s and 2p): 8 electrons
- Third level (3s, 3p, and 3d): 18 electrons
- Add Protons and Neutrons: Determine the number of protons (atomic number) and neutrons (mass number - atomic number) and place them in the nucleus.
Example: Drawing a Bohr Model Diagram for Oxygen (O)
- Step 1: Draw a small circle for the nucleus.
- Step 2: Add two energy levels (first and second levels) around the nucleus.
- Step 3: Place 2 electrons in the first level and 6 electrons in the second level.
- Step 4: Determine the number of protons (8) and neutrons (8) for oxygen and place them in the nucleus.
Solutions to Common Worksheet Questions
Question 1: What is the maximum number of electrons in the second energy level?
- Answer: 8 electrons
Question 2: How many protons and neutrons are in the nucleus of an atom of lithium (Li)?
- Answer: 3 protons and 4 neutrons
Question 3: Draw the Bohr model diagram for carbon ©.
- Answer:
- Nucleus: 6 protons and 6 neutrons
- Energy Levels: Two energy levels
- Electrons: 2 electrons in the first level and 4 electrons in the second level
Notes
📝 Note: The Bohr model is a simplified representation of the atom and does not accurately depict the complex behavior of electrons in atoms.
Key Points to Remember
- The Bohr model is a fundamental concept in understanding atomic structure.
- Energy levels or shells are the regions around the nucleus where electrons are found.
- Electrons occupy specific energy levels, and each level has a specific capacity.
As we have seen, the Bohr model diagram is a powerful tool for visualizing the structure of atoms. By understanding its components and how to draw it, we can gain a deeper insight into the world of atomic physics.
In conclusion, the Bohr model diagram is a crucial concept in chemistry and physics that helps us understand the structure of atoms. By mastering the skills to draw and interpret the Bohr model diagram, we can better comprehend the behavior of atoms and their interactions.
What is the main limitation of the Bohr model?
+The Bohr model does not accurately depict the complex behavior of electrons in atoms and does not account for the electron spin and orbital shapes.
How many electrons can the first energy level hold?
+The first energy level can hold a maximum of 2 electrons.
What is the difference between protons and neutrons?
+Protons have a positive charge, while neutrons have no charge. Both are found in the nucleus of an atom.
Related Terms:
- Bohr Model diagram worksheet pdf
- Bohr Model diagrams Answer Key
- Bohr Model diagrams worksheet
- Bohr model Worksheet Doc
- Bohr model Practice