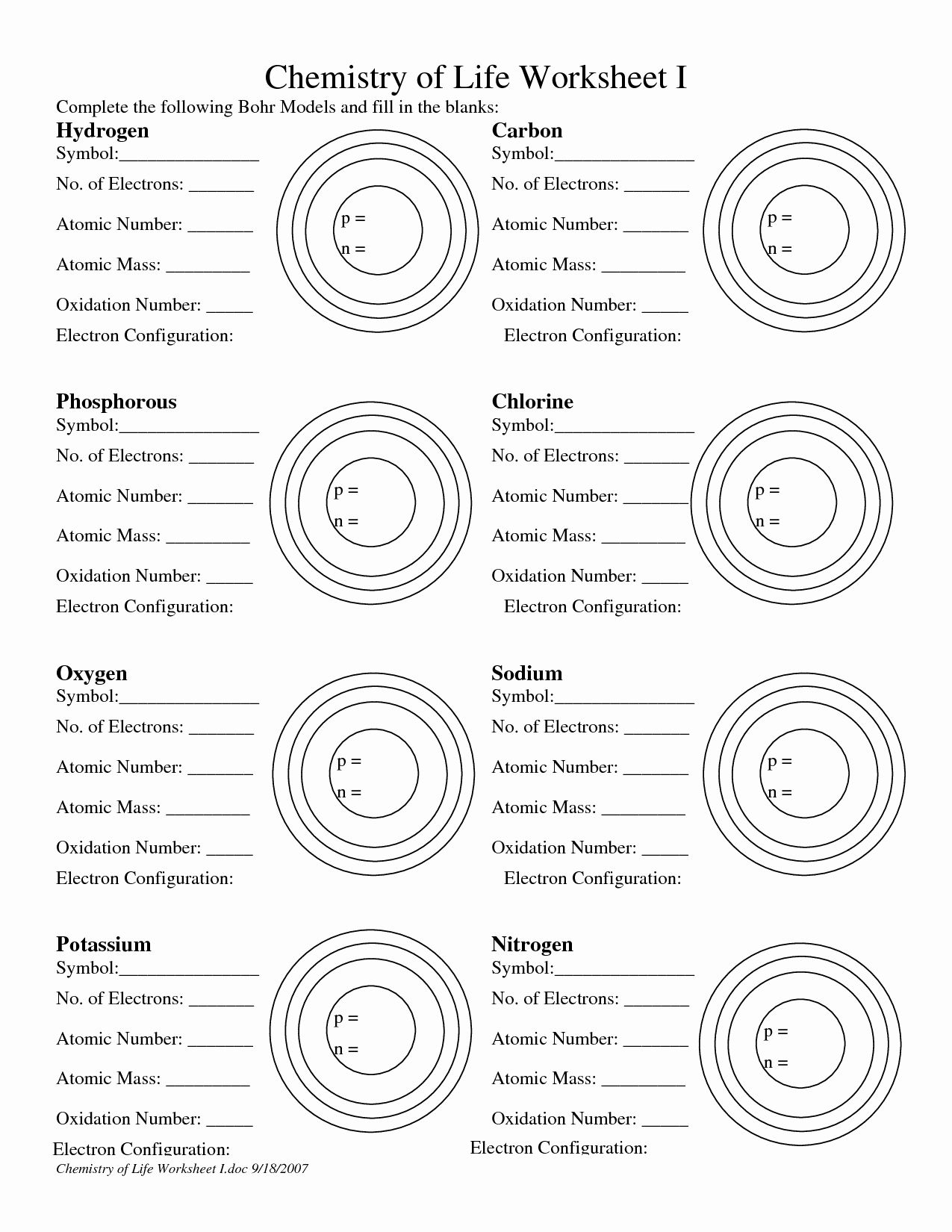
Bohr Atomic Model Worksheet Made Easy

Understanding the Bohr Atomic Model
The Bohr atomic model is a fundamental concept in chemistry and physics that explains the structure of an atom. Developed by Niels Bohr in 1913, this model revolutionized the way we understand the behavior of electrons within an atom. In this article, we will delve into the details of the Bohr atomic model, its key features, and how it can be applied to solve problems.
Key Features of the Bohr Atomic Model
The Bohr atomic model consists of several key features that distinguish it from other atomic models:
- Nucleus: The nucleus is the central part of the atom, containing protons and neutrons.
- Electron Shells: Electron shells, also known as energy levels, are the regions around the nucleus where electrons are found.
- Energy Levels: Energy levels are the specific regions within an electron shell where electrons can be found.
- Electron Jump: Electrons can jump from one energy level to another by absorbing or emitting energy.
Understanding Energy Levels
Energy levels are a crucial aspect of the Bohr atomic model. Each energy level has a specific capacity, and electrons can only occupy specific energy levels. The energy levels are designated by the principal quantum number (n), which can take values from 1 to infinity.
| Energy Level | Principal Quantum Number (n) | Electron Capacity |
|---|---|---|
| 1st Energy Level | 1 | 2 |
| 2nd Energy Level | 2 | 8 |
| 3rd Energy Level | 3 | 18 |
Electron Configuration
Electron configuration is the arrangement of electrons within an atom. To determine the electron configuration, we need to follow the Aufbau principle and the Pauli Exclusion Principle.
- Aufbau Principle: Electrons occupy the lowest available energy levels.
- Pauli Exclusion Principle: Each energy level can hold a maximum of two electrons with opposite spins.
🔍 Note: The electron configuration is written in the format of 1s² 2s² 2p⁶, where the number represents the energy level, the letter represents the type of orbital (s, p, d, or f), and the superscript represents the number of electrons in that orbital.
Bohr Atomic Model Worksheet
Now that we have covered the basics of the Bohr atomic model, let’s practice with a worksheet.
Part 1: Multiple Choice Questions
- What is the principal quantum number of the 3rd energy level? a) 1 b) 2 c) 3 d) 4
Answer: c) 3
- What is the electron capacity of the 2nd energy level? a) 2 b) 8 c) 18 d) 32
Answer: b) 8
Part 2: Short Answer Questions
- Describe the difference between the Aufbau principle and the Pauli Exclusion Principle.
Answer: The Aufbau principle states that electrons occupy the lowest available energy levels, while the Pauli Exclusion Principle states that each energy level can hold a maximum of two electrons with opposite spins.
- Write the electron configuration of the element oxygen (atomic number 8).
Answer: 1s² 2s² 2p⁴
Part 3: Long Answer Questions
- Explain the concept of electron jump in the Bohr atomic model.
Answer: Electron jump refers to the process by which an electron can move from one energy level to another by absorbing or emitting energy. This occurs when an electron gains or loses energy, allowing it to jump to a higher or lower energy level.
- Describe the significance of the Bohr atomic model in understanding the structure of an atom.
Answer: The Bohr atomic model provides a fundamental understanding of the structure of an atom, including the arrangement of electrons within energy levels. It has been widely used to explain the behavior of electrons and has led to numerous breakthroughs in chemistry and physics.
Conclusion
In conclusion, the Bohr atomic model is a fundamental concept in chemistry and physics that provides a comprehensive understanding of the structure of an atom. By understanding the key features of the Bohr atomic model, including energy levels, electron shells, and electron configuration, we can solve problems and explain the behavior of electrons within an atom.
What is the principal quantum number of the 1st energy level?
+The principal quantum number of the 1st energy level is 1.
What is the electron capacity of the 3rd energy level?
+The electron capacity of the 3rd energy level is 18.
What is the electron configuration of the element carbon (atomic number 6)?
+The electron configuration of the element carbon is 1s² 2s² 2p².