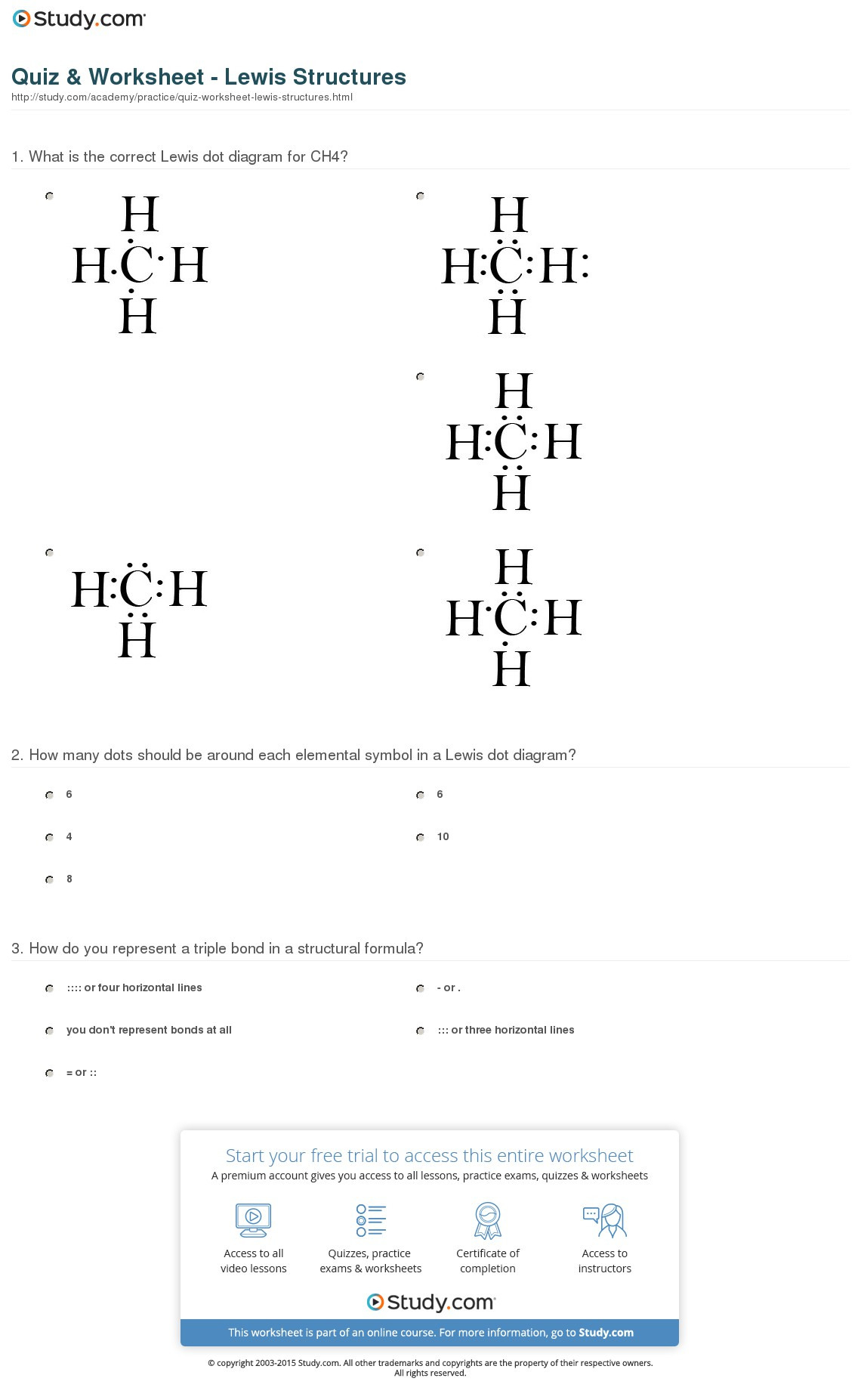
7 Ways to Master Limiting Reagent Worksheet Answers

Understanding Limiting Reagents: A Key to Mastering Chemistry
Limiting reagents are a fundamental concept in chemistry, and mastering them is crucial for solving various problems in stoichiometry. A limiting reagent is a reactant that is completely consumed in a chemical reaction, determining the amount of product that can be formed. In this article, we will explore seven ways to master limiting reagent worksheet answers, helping you to become proficient in this essential chemistry concept.
1. Understand the Concept of Limiting Reagents
To master limiting reagent worksheet answers, it’s essential to understand the concept of limiting reagents thoroughly. A limiting reagent is the reactant that is present in the least amount required for the reaction to occur. This means that even if other reactants are present in excess, the reaction will stop when the limiting reagent is completely consumed.
Key Takeaway:
- The limiting reagent determines the amount of product that can be formed in a chemical reaction.
2. Learn to Identify the Limiting Reagent
To identify the limiting reagent, you need to calculate the mole ratio of the reactants and compare it to the mole ratio required by the balanced equation. The reactant with the smallest mole ratio is the limiting reagent.
Example:
Calculate the limiting reagent in the reaction:
2Na + Cl2 → 2NaCl
Mole Ratio of Reactants:
- Na: 2 mol
- Cl2: 1 mol
Mole Ratio Required by Balanced Equation:
- Na:Cl2 = 2:1
In this example, Cl2 is the limiting reagent because it has the smallest mole ratio.
Key Takeaway:
- To identify the limiting reagent, calculate the mole ratio of the reactants and compare it to the mole ratio required by the balanced equation.
3. Practice with Simple Problems
Practicing with simple problems is essential to master limiting reagent worksheet answers. Start with simple stoichiometry problems and gradually move on to more complex ones. Make sure to identify the limiting reagent in each problem.
Example:
A 25.0-g sample of calcium carbonate (CaCO3) is decomposed to produce calcium oxide (CaO) and carbon dioxide (CO2). If the reaction produces 15.0 g of CaO, what is the limiting reagent?
Solution:
- Calculate the mole ratio of CaCO3 and CaO.
- Compare the mole ratio to the mole ratio required by the balanced equation.
- Identify the limiting reagent.
Key Takeaway:
- Practicing with simple problems helps to build confidence and mastery of limiting reagent concepts.
4. Use a Systematic Approach
Using a systematic approach is crucial to master limiting reagent worksheet answers. Break down the problem into smaller steps, and make sure to identify the limiting reagent in each step.
Systematic Approach:
- Write the balanced equation.
- Calculate the mole ratio of the reactants.
- Compare the mole ratio to the mole ratio required by the balanced equation.
- Identify the limiting reagent.
Key Takeaway:
- A systematic approach helps to ensure accuracy and completeness in solving limiting reagent problems.
5. Check Your Units
Checking your units is essential to master limiting reagent worksheet answers. Make sure to use the correct units for each quantity, and convert between units as necessary.
Example:
A 50.0-g sample of sodium chloride (NaCl) is dissolved in water to produce a solution. If the solution contains 25.0 g of NaCl per liter, what is the molarity of the solution?
Solution:
- Convert the mass of NaCl from grams to moles.
- Calculate the volume of the solution in liters.
- Calculate the molarity of the solution.
Key Takeaway:
- Checking your units helps to ensure accuracy and completeness in solving limiting reagent problems.
6. Practice with More Complex Problems
Practicing with more complex problems is essential to master limiting reagent worksheet answers. Try to solve problems with multiple reactants and products, and make sure to identify the limiting reagent in each problem.
Example:
A 100.0-g sample of calcium carbonate (CaCO3) is decomposed to produce calcium oxide (CaO) and carbon dioxide (CO2). If the reaction produces 50.0 g of CaO and 20.0 g of CO2, what is the limiting reagent?
Solution:
- Calculate the mole ratio of CaCO3, CaO, and CO2.
- Compare the mole ratio to the mole ratio required by the balanced equation.
- Identify the limiting reagent.
Key Takeaway:
- Practicing with more complex problems helps to build mastery and confidence in solving limiting reagent problems.
7. Review and Reflect
Reviewing and reflecting on your work is essential to master limiting reagent worksheet answers. Review your mistakes, and try to understand where you went wrong. Reflect on your strengths and weaknesses, and make a plan to improve your skills.
Reflection Questions:
- What are my strengths and weaknesses in solving limiting reagent problems?
- How can I improve my skills in solving limiting reagent problems?
- What are some common mistakes I make in solving limiting reagent problems, and how can I avoid them?
Key Takeaway:
- Reviewing and reflecting on your work helps to build mastery and confidence in solving limiting reagent problems.
As you work through these seven steps, you’ll become more proficient in solving limiting reagent worksheet answers. Remember to practice regularly, review your mistakes, and reflect on your strengths and weaknesses. With time and effort, you’ll master limiting reagent concepts and become a proficient chemistry student.
What is a limiting reagent?
+A limiting reagent is a reactant that is completely consumed in a chemical reaction, determining the amount of product that can be formed.
How do I identify the limiting reagent?
+To identify the limiting reagent, calculate the mole ratio of the reactants and compare it to the mole ratio required by the balanced equation. The reactant with the smallest mole ratio is the limiting reagent.
What is the importance of limiting reagents in chemistry?
+Limiting reagents are crucial in chemistry because they determine the amount of product that can be formed in a chemical reaction. Understanding limiting reagents is essential for solving stoichiometry problems and predicting the outcome of chemical reactions.