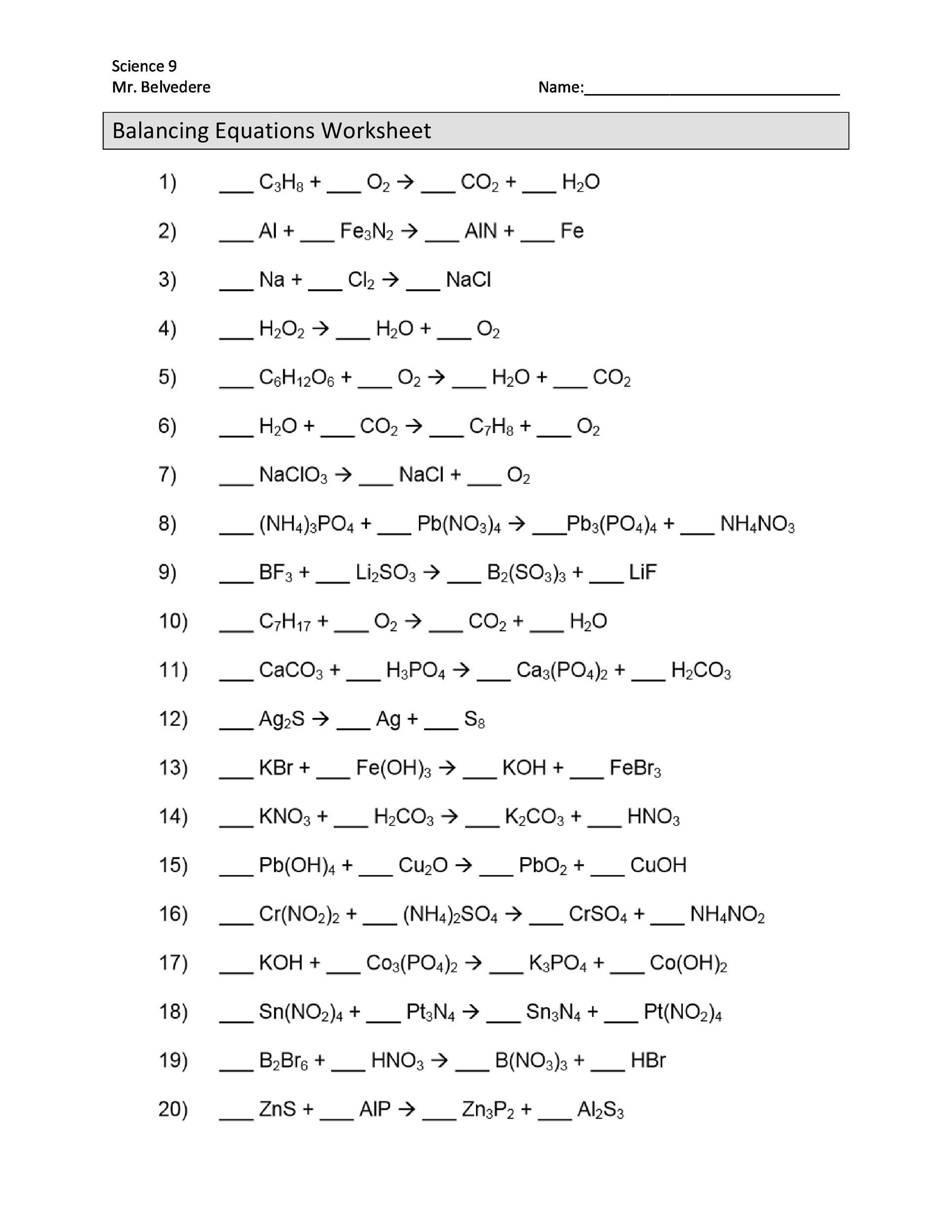
Bill Nye Atoms and Molecules Worksheet Answers

Atoms and molecules are the building blocks of matter, and understanding their structure and behavior is crucial for any science enthusiast. Bill Nye, also known as the “Science Guy,” has created a worksheet to help students learn about atoms and molecules. Here are the answers to the worksheet:
Section 1: Multiple Choice
- What is the smallest unit of matter that still retains the properties of an element? a) Molecule b) Compound c) Atom d) Mixture
Answer: c) Atom
- Which of the following is a characteristic of an atom? a) It has a definite shape and volume b) It has a fixed position in space c) It is the smallest unit of matter that still retains the properties of an element d) It is a mixture of two or more elements
Answer: c) It is the smallest unit of matter that still retains the properties of an element
- What is the term for the number of protons in an atom’s nucleus? a) Atomic number b) Mass number c) Electron number d) Proton number
Answer: a) Atomic number
Section 2: Short Answer
- What is the difference between an atom and a molecule?
Answer: An atom is the smallest unit of matter that still retains the properties of an element, while a molecule is a group of two or more atoms that are chemically bonded together.
- Describe the structure of an atom.
Answer: An atom consists of a nucleus, which contains protons and neutrons, surrounded by electrons that orbit the nucleus.
Section 3: True or False
- True or False: Atoms are the smallest units of matter that can exist.
Answer: True
- True or False: Molecules are made up of only one type of atom.
Answer: False (Molecules can be made up of two or more different types of atoms.)
Section 4: Fill in the Blank
- The number of protons in an atom’s nucleus determines its _______________________.
Answer: atomic number
- The electrons in an atom are arranged in energy levels or _______________________.
Answer: shells
Section 5: Matching
Match the following terms with their definitions:
- Atom
- Molecule
- Compound
- Mixture
Definitions:
a) A group of two or more atoms that are chemically bonded together b) A substance that is made up of two or more different elements c) A physical combination of two or more substances d) The smallest unit of matter that still retains the properties of an element
Answers:
- d) The smallest unit of matter that still retains the properties of an element
- a) A group of two or more atoms that are chemically bonded together
- b) A substance that is made up of two or more different elements
- c) A physical combination of two or more substances
Section 6: Essay Question
Describe the difference between an ionic bond and a covalent bond. Provide examples of each.
Answer: Ionic bonds are formed when one or more electrons are transferred between atoms, resulting in the formation of ions with opposite charges that attract each other. Covalent bonds, on the other hand, are formed when two or more atoms share one or more pairs of electrons to form a molecule. Examples of ionic bonds include table salt (sodium chloride) and calcium carbonate, while examples of covalent bonds include water (H2O) and methane (CH4).
Additional Notes
- Atoms are the building blocks of matter, and they are the smallest units of matter that still retain the properties of an element.
- Molecules are groups of two or more atoms that are chemically bonded together.
- Compounds are substances that are made up of two or more different elements.
- Mixtures are physical combinations of two or more substances.
I hope this helps! Let me know if you have any questions or need further clarification. 😊
Related Terms:
- Elements occur naturally