Balancing Chemical Equations Made Easy: Worksheet and Answers
Chemical Equations: A Fundamental Concept in Chemistry
Chemical equations are a crucial part of chemistry, as they provide a concise way to represent chemical reactions. A chemical equation is a symbolic representation of a chemical reaction, where reactants are transformed into products. Balancing chemical equations is a critical skill that chemists and chemistry students need to master. In this article, we will provide a comprehensive guide on how to balance chemical equations, along with a worksheet and answers to help you practice.
Why Balance Chemical Equations?
Balancing chemical equations is essential for several reasons:
- Conservation of Mass: The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. Balancing chemical equations ensures that the number of atoms of each element is the same on both the reactant and product sides.
- Accurate Representation: Balancing chemical equations provides an accurate representation of the chemical reaction, allowing chemists to predict the products and reactants involved.
- Quantitative Analysis: Balanced chemical equations enable chemists to perform quantitative analysis, such as calculating the amount of reactants required or the yield of products.
Step-by-Step Guide to Balancing Chemical Equations
Balancing chemical equations involves a few simple steps:
- Write the Unbalanced Equation: Write the chemical equation with the reactants on the left and the products on the right.
- Count the Atoms: Count the number of atoms of each element on both the reactant and product sides.
- Balance the Atoms: Balance the atoms by adding coefficients (numbers in front of the formulas of reactants or products) to ensure that the number of atoms of each element is the same on both sides.
- Check the Balance: Check the balance by counting the atoms again.
👍 Note: When balancing chemical equations, it's essential to balance the atoms of each element separately. Start with elements that appear only once on each side of the equation.
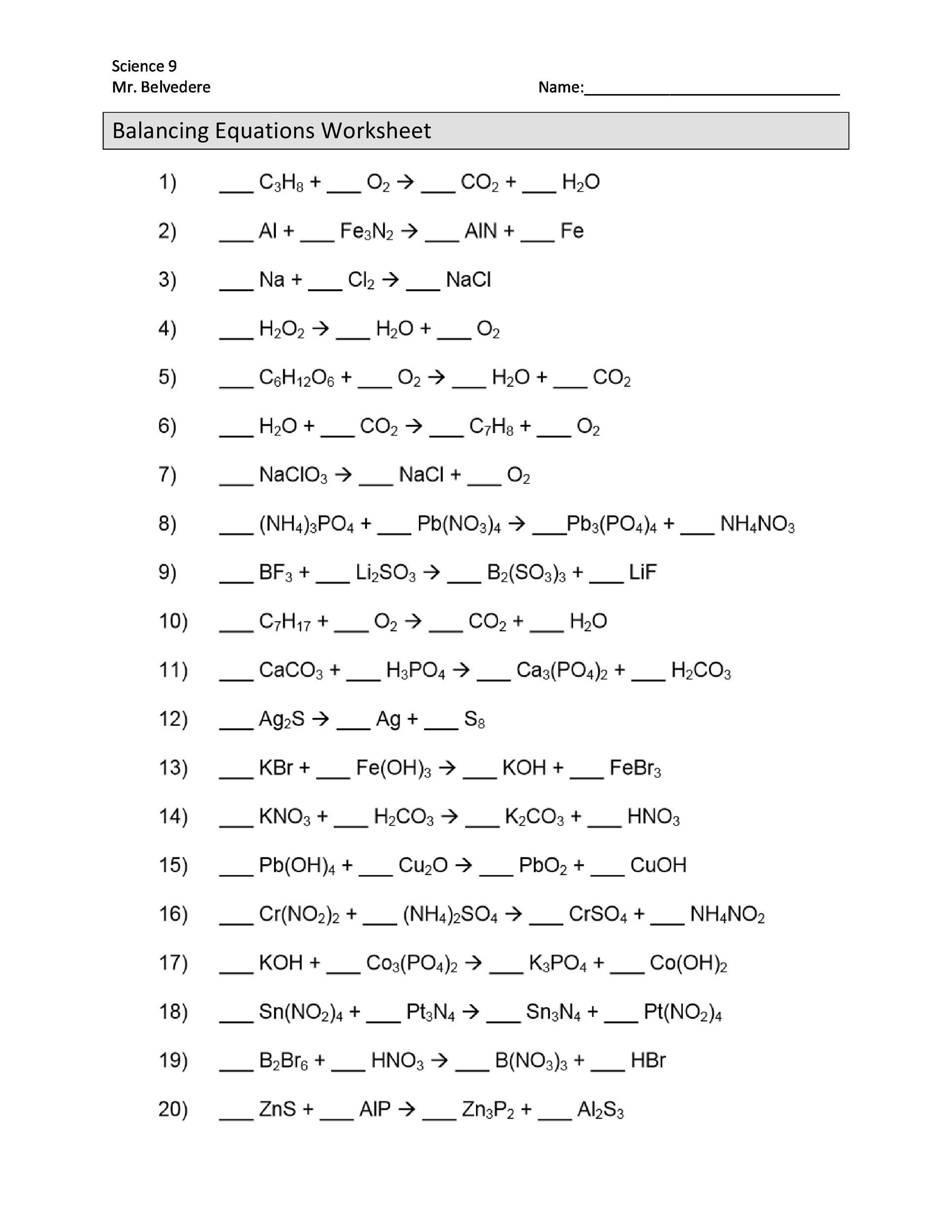
Worksheet: Balancing Chemical Equations
Practice balancing chemical equations with the following worksheet:
- Ca + O2 → CaO
- 2H2 + O2 → 2H2O
- Fe + CuSO4 → FeSO4 + Cu
- 2Na + Cl2 → 2NaCl
- 2Al + Fe2O3 → 2Fe + Al2O3
Answers
- 2Ca + O2 → 2CaO
- 2H2 + O2 → 2H2O (already balanced)
- Fe + CuSO4 → FeSO4 + Cu (already balanced)
- 2Na + Cl2 → 2NaCl (already balanced)
- 2Al + Fe2O3 → 2Fe + Al2O3 (already balanced)
📝 Note: When checking your answers, make sure to count the atoms of each element on both sides of the equation to ensure that they are balanced.
Common Mistakes to Avoid
When balancing chemical equations, it’s essential to avoid the following common mistakes:
- Changing the Formulas: Never change the formulas of the reactants or products to balance the equation. Instead, add coefficients to balance the atoms.
- Balancing with Subscripts: Avoid balancing the equation by changing the subscripts (small numbers within the formulas). Instead, add coefficients to balance the atoms.
- Forgetting to Check: Always check the balance by counting the atoms of each element on both sides of the equation.
Conclusion
Balancing chemical equations is a fundamental skill in chemistry that requires attention to detail and practice. By following the step-by-step guide and practicing with the worksheet, you can master the skill of balancing chemical equations. Remember to avoid common mistakes and always check the balance by counting the atoms of each element on both sides of the equation.
What is the purpose of balancing chemical equations?
+
Balancing chemical equations ensures that the number of atoms of each element is the same on both the reactant and product sides, providing an accurate representation of the chemical reaction.
How do I balance chemical equations?
+
Balance chemical equations by following the step-by-step guide: write the unbalanced equation, count the atoms, balance the atoms by adding coefficients, and check the balance.
What is the most common mistake when balancing chemical equations?
+
Changing the formulas of the reactants or products to balance the equation is the most common mistake. Instead, add coefficients to balance the atoms.
Related Terms:
- Chemistry balancing chemical equations worksheet
- balancing-chemical-equations-worksheet-1-qp.pdf answers