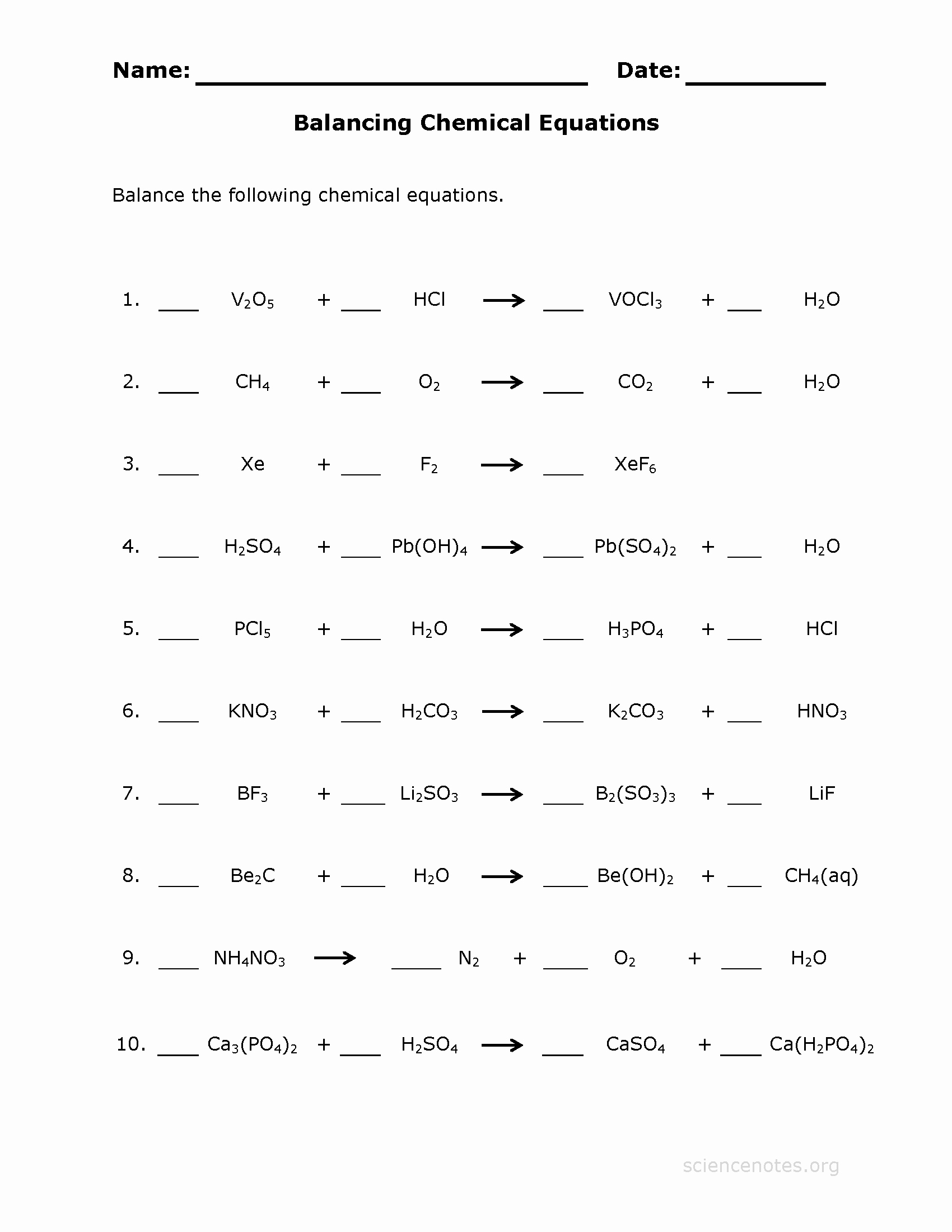
Balancing Equations Worksheet Answers Made Easy
Mastering the Art of Balancing Equations: A Step-by-Step Guide
Balancing chemical equations is a fundamental concept in chemistry that can be challenging for many students. It requires a deep understanding of the chemical reaction, the reactants, and the products involved. In this article, we will break down the process of balancing equations into simple, manageable steps, and provide examples and answers to help you master this essential skill.
What is a Balanced Equation?
A balanced equation is a chemical equation where the number of atoms of each element is equal on both the reactant and product sides. This is achieved by adding coefficients (numbers in front of the formulas of reactants or products) to balance the equation. The goal is to have the same number of atoms of each element on both sides of the equation.
Step 1: Write the Unbalanced Equation
The first step in balancing an equation is to write the unbalanced equation. This involves writing the formulas of the reactants and products, with an arrow (→) pointing from the reactants to the products.
Example: Hydrogen gas reacts with oxygen gas to form water.
H₂ + O₂ → H₂O
Step 2: Count the Atoms
Next, count the number of atoms of each element on both the reactant and product sides.

| Element | Reactants | Products |
|---|---|---|
| H | 2 | 2 |
| O | 2 | 1 |
Step 3: Balance the Atoms
To balance the atoms, add coefficients to the reactants or products. Start with the elements that appear only once on each side of the equation.
In this example, oxygen is not balanced. To balance oxygen, add a coefficient of 2 in front of H₂O.
H₂ + O₂ → 2H₂O
Now, re-count the atoms:
| Element | Reactants | Products |
|---|---|---|
| H | 2 | 4 |
| O | 2 | 2 |
The oxygen atoms are now balanced, but the hydrogen atoms are not.
Step 4: Balance the Remaining Atoms
To balance the hydrogen atoms, add a coefficient of 2 in front of H₂.
2H₂ + O₂ → 2H₂O
Now, re-count the atoms:
| Element | Reactants | Products |
|---|---|---|
| H | 4 | 4 |
| O | 2 | 2 |
The equation is now balanced!
Example 2: Balancing a More Complex Equation
Consider the reaction between sodium and chlorine to form sodium chloride.
Na + Cl₂ → NaCl
Follow the same steps as before:
- Write the unbalanced equation.
- Count the atoms.
- Balance the atoms.
| Element | Reactants | Products |
|---|---|---|
| Na | 1 | 1 |
| Cl | 2 | 1 |
To balance the chlorine atoms, add a coefficient of 2 in front of NaCl.
Na + Cl₂ → 2NaCl
However, this adds an extra sodium atom on the product side. To balance the sodium atoms, add a coefficient of 2 in front of Na.
2Na + Cl₂ → 2NaCl
The equation is now balanced!
👍 Note: When balancing equations, it's essential to check your work by re-counting the atoms after each step. This ensures that the equation is balanced and accurate.
Tips and Tricks for Balancing Equations
- Always start by balancing elements that appear only once on each side of the equation.
- Use coefficients to balance atoms, rather than subscripts (numbers within a formula).
- Check your work by re-counting the atoms after each step.
- Practice, practice, practice! The more you practice balancing equations, the more comfortable you’ll become with the process.
Conclusion
Balancing chemical equations is a fundamental skill in chemistry that requires patience, practice, and attention to detail. By following the steps outlined in this article, you’ll be well on your way to mastering the art of balancing equations. Remember to take your time, check your work, and practice regularly to become proficient in this essential skill.
What is the purpose of balancing chemical equations?
+The purpose of balancing chemical equations is to ensure that the number of atoms of each element is equal on both the reactant and product sides. This is essential for accurately representing chemical reactions and predicting the quantities of reactants and products involved.
What is the difference between a coefficient and a subscript?
+A coefficient is a number placed in front of a formula to balance an equation, while a subscript is a number within a formula that indicates the number of atoms of an element in a molecule. Coefficients are used to balance equations, while subscripts are used to represent the structure of molecules.
How can I check if an equation is balanced?
+To check if an equation is balanced, count the number of atoms of each element on both the reactant and product sides. If the number of atoms is equal on both sides, the equation is balanced. If not, adjust the coefficients to balance the equation.