Empirical and Molecular Formula Practice Worksheet Answers
Empirical and Molecular Formula Practice Worksheet Answers
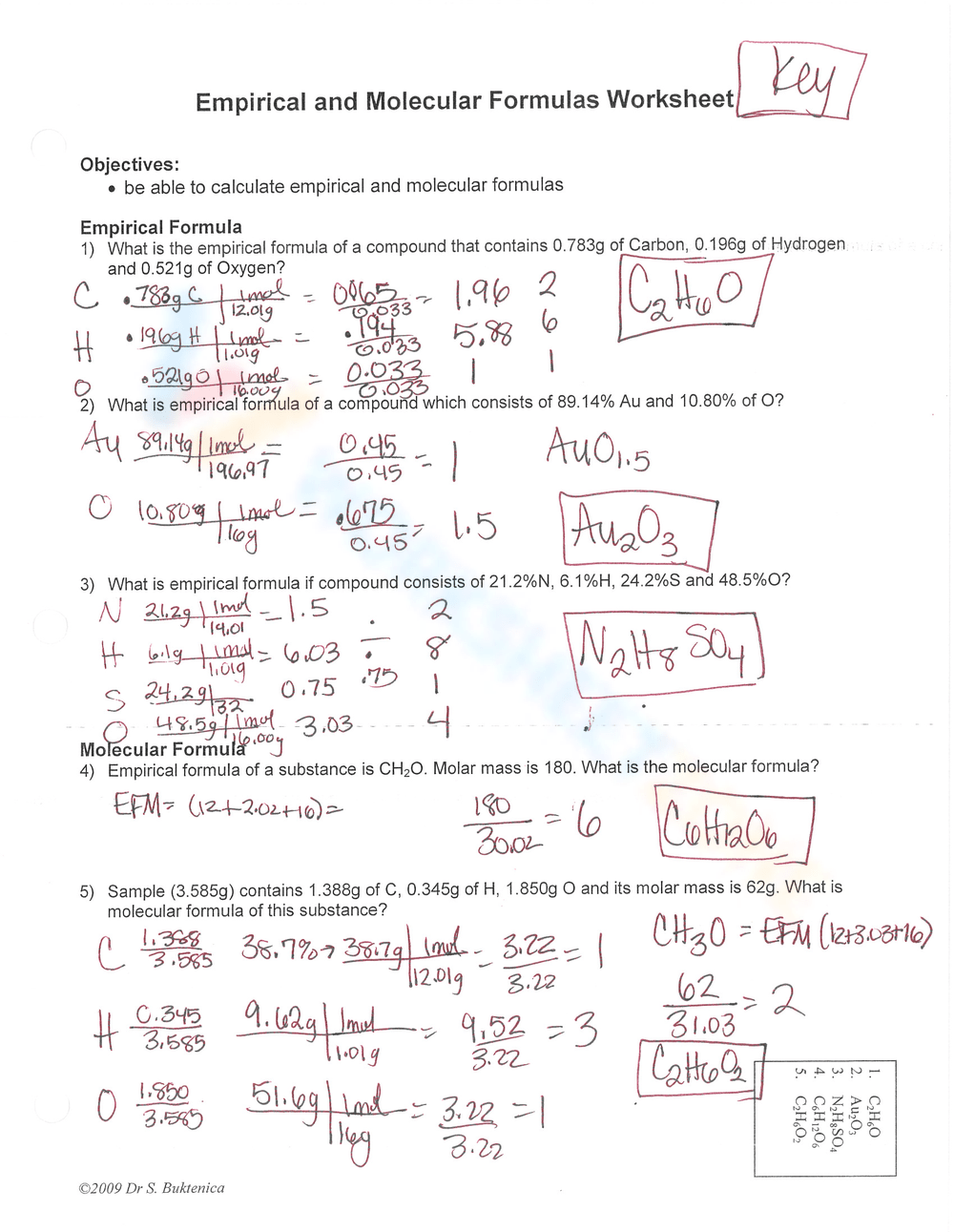
The empirical formula and molecular formula are two important concepts in chemistry that help us understand the composition of molecules. In this practice worksheet, we will go through some exercises to help you understand how to calculate empirical and molecular formulas.
What is an Empirical Formula?
An empirical formula is the simplest whole-number ratio of atoms of each element present in a compound. It is calculated based on the percentage composition of the compound.
What is a Molecular Formula?
A molecular formula is the actual number of atoms of each element present in a molecule. It is calculated based on the empirical formula and the molecular weight of the compound.
Practice Problems
Here are some practice problems to help you understand how to calculate empirical and molecular formulas:
Problem 1
A compound has the following percentage composition: carbon (40%), hydrogen (6.67%), and oxygen (53.33%). Calculate the empirical formula of the compound.
🔍 Note: To calculate the empirical formula, we need to divide the percentage composition of each element by its atomic mass and then divide by the smallest number of moles.

| Element | Percentage Composition | Atomic Mass | Moles |
|---|---|---|---|
| Carbon (C) | 40% | 12.01 g/mol | 3.33 mol |
| Hydrogen (H) | 6.67% | 1.01 g/mol | 6.61 mol |
| Oxygen (O) | 53.33% | 16.00 g/mol | 3.33 mol |
The empirical formula is CH2O.
Problem 2
A compound has the empirical formula CH2O and a molecular weight of 60.05 g/mol. Calculate the molecular formula of the compound.
🔍 Note: To calculate the molecular formula, we need to multiply the empirical formula by a factor that will give us the molecular weight.
The molecular formula is C2H4O2.
More Practice Problems
Here are some more practice problems to help you understand how to calculate empirical and molecular formulas:
Problem 3
A compound has the following percentage composition: nitrogen (35.51%), hydrogen (5.03%), and oxygen (59.46%). Calculate the empirical formula of the compound.
| Element | Percentage Composition | Atomic Mass | Moles |
|---|---|---|---|
| Nitrogen (N) | 35.51% | 14.01 g/mol | 2.53 mol |
| Hydrogen (H) | 5.03% | 1.01 g/mol | 5.00 mol |
| Oxygen (O) | 59.46% | 16.00 g/mol | 3.71 mol |
The empirical formula is NH5O2.
Problem 4
A compound has the empirical formula NH5O2 and a molecular weight of 135.14 g/mol. Calculate the molecular formula of the compound.
🔍 Note: To calculate the molecular formula, we need to multiply the empirical formula by a factor that will give us the molecular weight.
The molecular formula is N2H10O4.
What is the difference between empirical and molecular formulas?
+The empirical formula is the simplest whole-number ratio of atoms of each element present in a compound, while the molecular formula is the actual number of atoms of each element present in a molecule.
How do I calculate the empirical formula of a compound?
+To calculate the empirical formula, you need to divide the percentage composition of each element by its atomic mass and then divide by the smallest number of moles.
How do I calculate the molecular formula of a compound?
+To calculate the molecular formula, you need to multiply the empirical formula by a factor that will give you the molecular weight.
In conclusion, calculating empirical and molecular formulas is an important skill in chemistry that helps us understand the composition of molecules. With practice, you can master this skill and become proficient in calculating empirical and molecular formulas.
Related Terms:
- Empirical Formulas Worksheet 1